5.2.1 Histopathological and Immunohistopathological (IHC) examination
For Histopathological/IHC examination, pieces of intestine are fixed by immersion in buffered formalin solution and embedded in a medium for sectioning (generally paraplast).
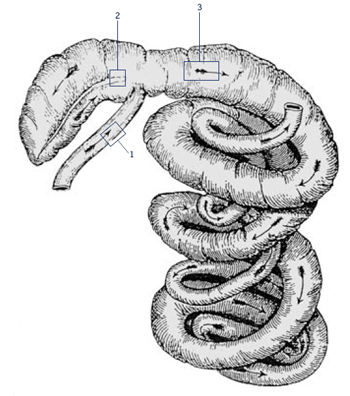
Picture 5.2.1 a
Sampling sites for histopathology, immunohistochemistry
and PCR.
Take samples for PCR and histopathology/IHC at 10cm before ileal-caecal entrance (number 1), 5cm at the base of the caecum (number 2), and at 10cm of the upper proximal colon (number 3). Place samples for histopathology in the jar with formaldehyde and avoid any damage to the very sensitive mucosal surface. Use three separate plastic containers for PCR tissues, which need to be placed on ice immediately. Make sure, all containers are labelled with a permanent marker, to be able to identify animal number and site/origin of tissue.
Histopathological findings vary but in all cases the baseline is as follows: In small intestine villi are short or completely lost and the surface epithelium is cuboidal or flattened. Lymphatics may be distended. The mucosa is thickened by proliferating crypts, which is the same in caecum and colon. There are four to five layers of intensely stained epithelial cells and goblet cells are sparse or missing. The mitotic rate in crypts is increased and in some crypt lumina cellular debris is accumulating(see pict 5.2.1 b). In comparison to an uninfected mucosa (see pict. 5.2.1 c), there is a mild to moderate inflammatory response in the lamina propria, sometimes even extending in deeper layers.
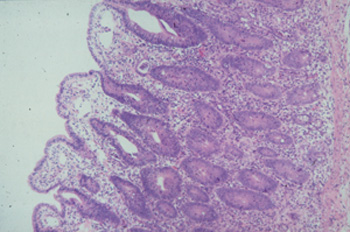
Picture 5.2.1 b (by J. Pohlenz)
Porcine ileum with PIA: Short intestinal villi covered with flat to cuboidal epithelial cells, sub epithelial oedema and proliferating crypts. There are two to three layers of crypt epithelial cells, loss of goblet cells, increased numbers of mitotic figures and intraepithelial cells, and a mild to moderate inflammatory reaction in the lamina propria (~125x).
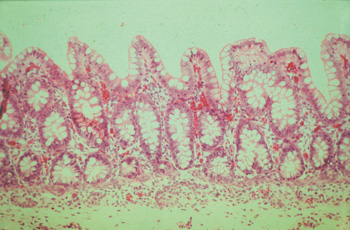
Picture 5.2.1 c (by J. Pohlenz)
Ileum of a healthy fattening pig with regular formation of intestinal villi and crypts, containing
a fair number of goblet cells (125x).
In more advanced stages of severe Necroprolife-rative Enteritis, there is branching of crypts and obvious crypt irregularity with severe necrosis on the surface (see pict. 5.2.1 d).
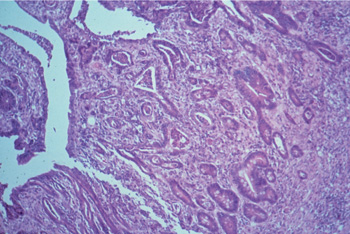
Picture 5.2.1 d (by J. Pohlenz)
Ileum of a pig with NPE. Irregularly proliferating
branching crypts, some of them filled with cellular debris, necrosis on surface (125x).
Lawsonia intracellularis cannot be detected by H&E stain. IHC does, reveal a specific reaction of the intracellular organism. The monoclonal antibodies used for Immunohistochemistry can be labeled for a direct in situ immunoperoxidase staining (see pict. 5.2.1 e) or immunofluorescense staining (see pict. 5.2.1 g). This specific reaction is detectable in most of the cases at the luminal aspect of the infected crypt cells, and is occasionally present in macrophages of the lamina propria. In a higher power magnification, there are increased numbers of intraepithelial lymphocytes and a few apoptotic figures recognizable (pict. 5.2.1 f).
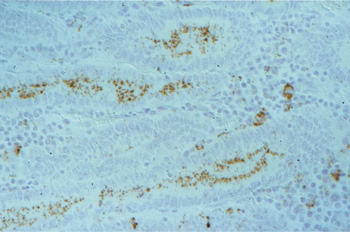
Picture 5.2.1 e (by J. Pohlenz)
IHC (immunoperoxidase) of a section of ileum with PIA: dark brown bacteria in enterocytes of crypts and in macrophages of the lamina propria (250x).
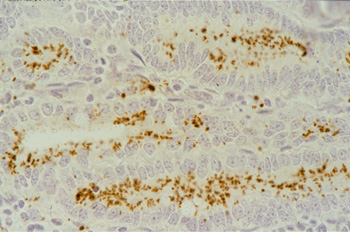
Picture 5.2.1 f (by J. Pohlenz)
Higher magnification of Picture 5.2.1e Lawsonia intracellularis predominantly located intracellular in the luminal aspect of the crypt enterocytes (500x).
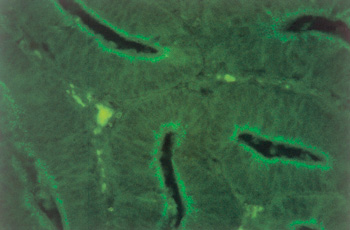
Picture 5.2.1 g (by M. Dünser)
Detection of Lawsonia intracellularis in ileum crypts by in situ hybridization using a fluorescein-labeled immunofluorescence assay (400x).
This helpful demonstration of the causative agent was not possible until monoclonal antibodies became available. Lawsonia intracellularis do stain black, when a silver stain (Warthin/Starry) is used. As they are acid-fast bacteria Lawsonia appear red against a blue background in the non-specific Ziehl-Nielsen staining (see pict. 5.2.1 h).
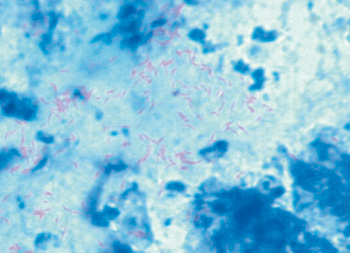
Picture 5.2.1 h (by M. Dünser)
Ziehl-Nielsen staining of Lawsonia intracellularis.
© Boehringer Ingelheim Animal Health GmbH, 2006
All rights reserved. No part of this Technical Manual 3.0 may be reproduced or transmitted in any form or by any means, electronic or photocopy, without permission in writing from Boehringer Ingelheim Animal Health GmbH.






