8.2 What are the experiences with Enterisol® Ileitis in the USA, Canada and Mexico?
USA:
Study 1: Five large-scale field trials with about 120,400 pigs were conducted in separate production systems across the USA to assess the biologic and economic performance of Enterisol® Ileitis.
Trials 1 to 4 had contemporary controls while trial 5 used historical controls in a before-after comparison as part of a continuous improvement project. In each case ‘barn’ was the experimental unit and pigs were vaccinated in the mid-to-late nursery phase (6 to 10 weeks of age) in these multi-site systems. Data in Table 8.2 a represents performance in the growing-finishing stages of production collectively compared across the 5 trials. A total of 159 barns and 120,444 pigs were included in these trials.
‘Control’ groups received the feed antibiotic medication programs in place at each production system at the time of study for disease control (including Ileitis) and growth promotion but were not vaccinated with Enterisol® Ileitis. Feed medications used included tylosin (5/5 trials), bacitracin (5/5), carbadox (3/5), and chlortetracycline (2/5) at various inclusion rates and schedules. In each case feed medications were used continuously throughout finishing in control groups. ‘Vaccinates’ received Enterisol® Ileitis vaccine but had their feed medication usage substantially reduced. The antibiotic feeding period (primarily Tylosin) was shortened by 3 to 9 weeks which resulted in conservatively estimated cost savings of $0.67 – $1.04 per pig. Vaccine cost was standardized at $1/pig for economic projections.
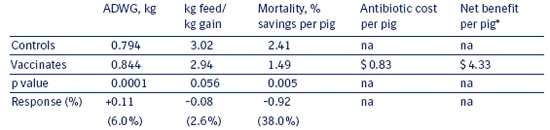
Table 8.2 a
Average response to Enterisol® Ileitis vaccination
with reduced feed medication compared
to continuous feed medication without vaccination across 5 trials.
* Assumes $88/100 kg market value, 25 kg placement weight, $120/ton feed cost, 125 days on feed for both groups; and $1/dose Enterisol® Ileitis cost and $.83/pig antibiotic cost savings for vaccinates. Only the statistically significant differences in ADWG, kg f/kg g, and mortality were used in the economic projection. Not included are differences in ad hoc group Ileitis treatment costs (which favored vaccinates) or a reduction in lightweight pigs (sort loss; which also favored vaccinates) because these data were not available from all studies.
Table 8.2 b shows the range of responses seen across the 5 individual trials as well as the effect on each parameter when analyzed collectively across all 5 trials (bottom row). Trial 5 did not have clinically apparent Ileitis in the presence of continuous feed medication and may be considered a subclinical Ileitis comparison. Medication savings were based on current market prices as of July 2003.
Eliminating the need for continuous feed medication recovered a large part of the standardized cost of vaccine. This situation is unique in that many interventions only add cost rather than providing some level of cost reduction. Some producers in the U.S. have successfully eliminated feed antibiotics (including growth promoters) all together from the finisher diets after proper vaccination.
Vaccination with Enterisol® Ileitis consistently improved ADWG, improved kg feed/kg gain and reduced mortality, even with reduced feed antibiotic usage, compared to continuously-medicated non-vaccinated groups. In summary, in over 120,000 pigs across 5 locations Enterisol® Ileitis provided:
- $4 Net Benefit per pig placed
- $0.83 feed antibiotic reduction per pig
Enterisol® Ileitis allows pork producers to win on both sides of the benefit to cost equation.
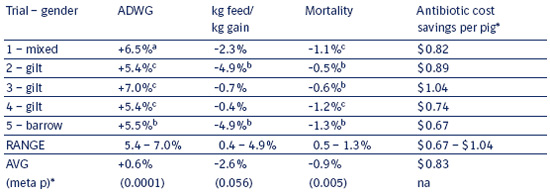
Table 8.2 b
Range of responses to Enterisol® Ileitis vaccination
and reduced feed medication compared
to continuous feed medication without
vaccination in five individual trials.
a p ≤ 0.07; b p ≤ 0.05; c p ≤ 0.01 for vaccinates vs. controls.
See individual trial summaries for actual p values.
* meta p is the p value collectively across the five trials for each parameter.
Study 2: In a recent study in a large swine production system in Minnesota/USA the performance of groups of finishing pigs continuously fed dietary antimicrobials was compared to those vaccinated with Enterisol® Ileitis and fed no finishing antimicrobials. (Nerem et al. 2006)
In this trial two treatment groups were replicated 11 times (22 total barns containing 28,765 pigs at 7 sites) with barn as the experimental unit. Vaccinates were on non-medicated feed during the last three weeks in the nursery to permit vaccination while the controls were on a standard feed medication protocol. Vaccinates received a single dose of the deep frozen form of Enterisol® Ileitis via their drinking water approximately two weeks prior to entering the finisher. Controls received 44 ppm tylosin phosphate continuously in feed throughout the finishing period (21 – 123kg). Vaccinates received no dietary antimicrobials during finishing.
The finishing performance of vaccinated pigs equalled that of continuously medicated pigs with US $ 0.50 less total health product costs per pig (Table 8.2 c). Use of 10 grams of dietary tylosin phosphate per pig was avoided by using vaccine for control of PE.
This study demonstrated that producers may have the option of eliminating finishing dietary antimicrobial use while reducing input costs and maintaining performance similar to continuously medicated, non-vaccinated pigs. Vaccine use has demonstrated improved finishing performance when added to dietary antimicrobial use, with the economic advantage expressed as improved productivity with equal input costs. As restrictions on antimicrobial use continue to increase, pork producers will need more options for controlling diseases like PE which have traditionally accounted for much of the need for antimicrobial use. Use of an effective vaccine to control PE is a biologically feasible, environmentally responsible and economically attractive alternative to continuous feeding of antimicrobials.

Table 8.2 c
Finishing performance of pigs vaccinated or medicated for control of proliferative enteropathy.
Canada:
The use of Enterisol® Ileitis in Canada is very successful deployed in breeding organisations, because many of them see problems with PHE with bloody diarrhoea and high mortality in gilts. Field observations on several hundred thousand gilts placed in recipient herds are used to estimate the efficacy and duration of immunity of Enterisol® Ileitis in vaccinated replacement gilts.
Records of Enterisol® Ileitis vaccinated replacement gilts from eight seedstock suppliers in Canada were compiled. Gilts, vaccinated in each of the years 2002, 2003, 2004 and 2005, were tracked for their survival, free of clinical Ileitis, in recipient herds. Historically, PHE in replacement gilts, with or without preventative treatment, had been common occurrences in most of these recipient herds.
Table 8.2 d documents Ileitis cases in Enterisol® Ileitis vaccinated gilts after entering recipient herds. In the forth year, as few as 6 animals on every 10,000 vaccinated displayed problems with Ileitis. The Ileitis breaks occurred usually in the first 2 – 6 months of the supplier starting to use the Enterisol® Ileitis vaccine and most often ceased to exist after this period in time.
Vaccinated gilts have now lived, without revaccination, for up to 3½ years in recipient herds without Ileitis breaks. Furthermore, over 200,000 of these gilts have survived at least 2 years without reports of Ileitis.
These field observations would suggest that the duration of immunity for Enterisol® Ileitis vaccinated gilts is at least 2 years and probably greater than 3½ years. This would adequately cover the entire productive life for most breeding stock in conventional herds. (Sanford 2006)
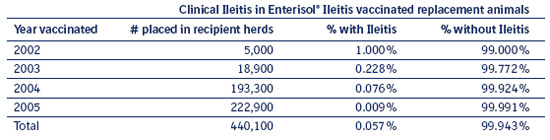
Table 8.2 d
Ileitis histories of Enterisol® Ileitis vaccinated
replacement animals after entering recipient herds.
Mexico:
In Mexico at least 80% of the farms have one animal positive by serology, leading to widespread in-feed antimicrobial use to control this disease. In a study a traditional feed medication programme was compared to the use of Enterisol® Ileitis combined with reduced strategic medication.
The study was performed in northern Mexico. The production system was a multiple-site farm with 4,000 sows. A total of 11 weekly production batches were evaluated; 7 control groups and 4 vaccinated groups.
Production parameters were evaluated using standard statistical process control methods. Criteria evaluated included: Average daily weight gain (ADWG), Feed efficiency (FE), Age at market, Weight at market, and Percent Culls.
Treatment procedures for each group were as follows:
The control group used a pulse feed medication program during the entire finishing phase:
- Tylosin 110 ppm/carbadox 55 ppm
(12 – 25 kg Body weight) - Tylosin 88 ppm/carbadox 27.5 ppm
(25 – 40 kg Body weight) - Tylosin 40 ppm/salinomycin 60 ppm
(40 – 60 skg Body weight)
Enterisol® Ileitis was administered to the vaccination group at 5 weeks of age in the nursery.
A pulse medication program was implemented to reduce the infection pressure during the onset of immunity period:
- Tylosin 88 ppm/carbadox 55 ppm
(25 – 40 kg Body weight) - Salinomycin 60 ppm
(40 – 60 kg Body weight).
The results in Table 8.2 e show that vaccinated pigs grow nearly 15% faster than conventionally medicated pigs.
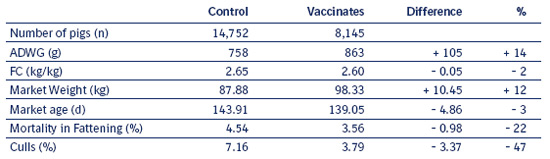
Table 8.2 e
Production Parameters and percentage of improvement
The results show a broad improvement in groups of vaccinated pigs. Even with a reduction of antimicrobial use, pigs grew faster, more consistently, and with nominally improved feed efficiency. The major economic benefits were average daily weight gain and cull percent.
Additionally, improvement in the intestinal health in all the vaccinated groups was noted, allowing pigs to manifest their genetic potential, growing faster with less antimicrobial use. This production benefit could allow producers to remain economically viable while still meeting consumer demands for pork produced with reduced use of antimicrobials. (Diaz and Chevez 2006).
© Boehringer Ingelheim Animal Health GmbH, 2006
All rights reserved. No part of this Technical Manual 3.0 may be reproduced or transmitted in any form or by any means, electronic or photocopy, without permission in writing from Boehringer Ingelheim Animal Health GmbH.






