3.3 How fast is the onset of infection and how long does it last?
Studies have been done showing the development and evolution of proliferative enteropathy lesions in both the hamster (Jacoby 1978, Johnson and Jacoby 1978) and pig (Guedes 2002). An electron microscopy study (Johnson and Jacoby 1978), using hamsters experimentally infected with affected intestinal mucosa homogenate, showed that the primary lesion was mucosal hyperplasia with progressive replacement of mature villi columnar absorptive cells by undifferentiated crypt-type cells. Those undifferentiated cells expanded onto villi walls from their normal location in crypts by day 10 post infection and reached the tips of the villi by day 14. Aggregates of an intracellular organism, now known to be L. intracellularis, were observed in the apical cytoplasm of crypt epithelium cells by day 5. Enterocyte hyperplasia could only be detected by day 10 using light microscopy examination of hematoxylin and eosin stained sections (Jacoby 1978). Enterocyte hyperplasia associated with the presence of L.intracellularis was observed for up to 40 days in hamsters challenged with intestinal mucosa homogenate (Frisk 1976, Jacoby 1978) from diseased animals.
Studies showing successful reproduction of proliferative enteropathy in pigs using pure culture of isolates of L.intracellularis (Mapother et al. 1987, McOrist et al. 1993, McOrist et al. 1994, McOrist et al. 1996, Smith and McOrist 1997, Knittel et al. 1998, Guedes and Gebhart 2003) as oral challenge inocula for conventional pigs or gnotobiotic pigs predosed with nonpathogenic enteric flora have consistently reproduced clinical signs and macroscopic lesions characteristic of proliferative enteropathy. Three weeks following oral inoculation with L.intracellularis, intracellular organisms, as well as enterocyte proliferation, could be identified in intestinal sections at necropsy.
Early lesions of proliferative enteropathy were studied in vivo in pigs and hamsters by electron microscopy. L.intracellularis in the crypt lumen associates with the cell membrane and enters the immature epithelial cell via an entry vacuole (McOrist et al. 1989). The vacuole breaks down and bacteria multiply freely within the cytoplasm. These infected epithelial cells continue to undergo mitosis, transmitting the organisms to daughter cells (McOrist et al 1995). Eventually, the bacteria are released from cytoplasmic extrusions on the epithelial cells on top of the villi or between crypts. Infection spreads to the entire ileum, distal jejunum, caecum, and colon. Propagation of infection occurs via invasion of epithelial cells into new crypts through the lumen or through the lamina propria.
Studies that examined the progression of gross and histologic lesions and the infection in pigs through the course of experimentally-produced proliferative enteropathy have been reported (Guedes 2002, Guedes and Gebhart 2003, Guedes 2004) (Figure 3.3 a and 3.3 b).
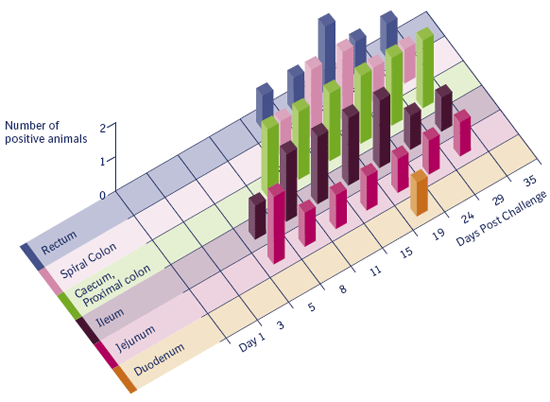
Figure 3.3 a
Number of pigs with Lawsonia intracellularis antigen labeling (ICH).
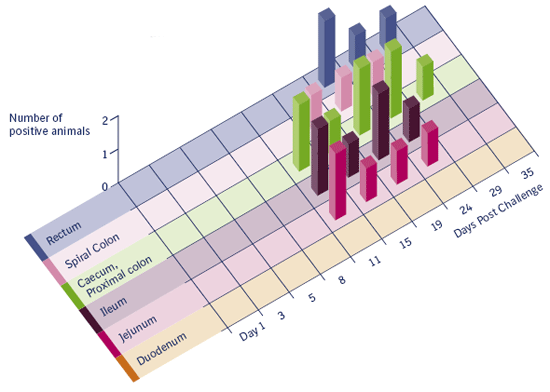
Figure 3.3 b
Number of pigs with hyperplastic enterocytes.
Figure 3.3 a and 3.3 b
Incidence of mucosal hyperplasia and bacterial antigen labeling in different intestinal segments in pigs experimentally
inoculated (2 pigs at each date) with pure culture of Lawsonia intracellularis (Guedes 2004). Both figures are clearly showing a transient infection in time through the intestines from proximal to distal.
Macroscopic lesions suggestive of proliferative enteropathy were first detected 11 days after infection. Histologic lesions characterized by enterocyte hyperplasia and reduction of goblet cells were first observed 11 days after inoculation. L. intracellularis antigen was first detected in the intestine by immunohistochemistry 5 days after inoculation. Positive staining, but no gross or histologic lesions, was detected on day 29. All pigs euthanized on day 35 were normal and negative by immunohistochemical staining. Conversely, one challenged pig was PCR positive on day 35 showing that, despite the nondetection of the bacterium by immunohistochemistry in examined intestinal fragments.
L. intracellularis antigen was not detected in any organ other than the intestines, lymph nodes and tonsils. It appears that L. intracellularis infection is limited to enterocytes and the bacterial antigen found in the lamina propria and mesenteric lymph nodes is probably due to the fact that the organisms are carried out by macrophages. Infection of enterocytes in the large intestine and rectum seems to occur later in the course of the disease and, consequently, the infection can be detected later in these locations. It appears that the small intestine is infected first and the bacteria shed from those sites infect lower levels of the gastrointestinal tract. Tonsil does not appear to be part of the bacterial pathogenesis but crypt cells may have L. intracellularis antigen in the cytoplasm.
© Boehringer Ingelheim Animal Health GmbH, 2006
All rights reserved. No part of this Technical Manual 3.0 may be reproduced or transmitted in any form or by any means, electronic or photocopy, without permission in writing from Boehringer Ingelheim Animal Health GmbH.




