



Genesus Global Technical Report
Genomic selection for disease resilience using measures of antibody (ab) response.
Disease is a major contributor to increased costs in the swine industry. For example, the cost estimated from the Porcine Reproductive and Respiratory Syndrome (PRRS) disease alone is $664 million annually to the US swine industry (year 2012 estimate), with 45 percent of this cost attributable to the breeding herd and 55 percent attributable to the grower/finisher herd (PRRS.com; Boehringer Ingelheim Vetmedica GmbH). The report breaks down the cost to a per pig basis, equating to $4.67 for every pig marketed in the US. If we consider the effects of other global swine diseases, the cost is much higher. Therefore, selection of pigs with improved disease resilience will significantly influence the swine industry`s ability to supply affordable pork products.
In a previous technical report, we shared Genesus’ perspective on genetic selection for healthier pigs (The Path to Genetically Healthier Pigs). Disease in swine is not likely to disappear. It is probable that mitigating the impacts of disease pressure will become more challenging due to geographical spread of currently identified pathogens, and mutation of existing strains into new.
Our intent is to identify pigs within Genesus breeds that are naturally more resilient to pathogen challenge and allow them to pass their desirable genes on to the next generation of selection candidates. This strategy results in the inclusion of an additional component to the breeding objective of Genesus purebred breeds, and a path toward genetic improvement for disease resilience.
One potential method of implementation is by placing selection emphasis on antibody response. The immune system elicits an immune response when an antigen is introduced. The level of immune response mounted within the individual can be measured from blood. Heritability is an estimation of the amount of control that genetics has in the expression of a phenotype and is estimable only when known pedigrees or genotypes accompany the phenotype. In a challenged environment, antibody response showed moderately high heritability (h2 = 0.45 ± 0.13) on Genesus grandparent females (Serão et al., 2014), which suggests this trait will respond to genetic selection.
In the same study, large and favorable genetic correlations (rg) between antibody response and reproductive outcomes were discovered, which were much higher than their phenotypic correlations (rp) (Table 1). A secondary study where the Genesus population was used in cross-validation supported these initial findings, reporting heritability with a range of 0.28 - 0.47, depending on the data used (Serão et al., 2016). A later study using a separate F1 female population reported a similar heritability for vaccine response (h2 = 0.34 ± 0.05) (Sanglard et al., 2020).
Together, these results indicate that antibody response to PRRS vaccine is under genetic control and can be used as a predictor of reproductive performance under PRRS challenge. These conclusions point to this approach as a potentially effective strategy to select pigs with improved disease resilience.
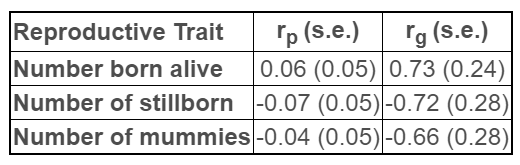
© Serão et al., 2014
The clean environment within nucleus herd settings does not present a challenge-type environment suitable for measuring resilience phenotypes, and vaccination for many diseases is not an option. However, vaccination is a common practice in commercial farms, and the timing often coincides with the timeline required to capture data for implementation of genetic selection. The isolation of incoming replacement gilts allows for the measurement of Ab response from vaccination, as incoming replacement gilts are typically health-tested to ensure no disease is present prior to introduction into the sow farm.
Previous research discovered that PRRS-infected and PRRS-vaccinated animals show similar antibody responses (Ellingson et al., 2010). The level of antibody elicited within an individual serve as the phenotype. Therefore, genetic selection under vaccine challenge will mirror selection under a direct challenge. Associations between Ab response and other reproductive and sow productivity traits are then estimated to establish the basis for genomic selection for disease resilience.
Obtaining phenotypes from environments outside the nucleus herd is important to the advancement of purebred populations. Genesus is acquiring genotype, phenotype, and pedigree information from commercial farms. Employing antibody response phenotypes in a genomic evaluation of purebred relatives provides an additional phenotype to aid in genetic improvement toward healthier pigs.
| References | ||||
|---|---|---|---|---|
| Ellingson, J. S., Weng Y., Layton S., Ciacci-Zanella J., Roof M.B. and Faaber K.S. | ||||
| (2010) | Vaccine efficacy of porcine reproductive and respiratory syndrome virus chimeras. Vaccine | 28:2679–2686. | ||
| Boehringer Ingelheim Vetmedica GmbH | ||||
| Global PRRS Solutions | PRRS.com | |||
| Sanglard L.P., Fernando R.L., Gray K.A., Linhares D.C., Dekkers J.C., Niederwerder M.C. & Serão N.V. | ||||
| (2020) | Genetic analysis of antibody response to porcine reproductive and respiratory syndrome vaccination as an indicator trait for reproductive performance in commercial sows. Frontiers in genetics | |||
| Serão N.V., Matika O., Kemp R.A., Harding J.C.S., Bishop S.C., Plastow G.S. & Dekkers J.C.M. | ||||
| (2014) | Genetic analysis of reproductive traits and antibody response in a PRRS outbreak herd. Journal of Animal Science | 92: (2905–21) | ||
| Serão, N. V., Kemp, R. A., Mote, B. E., Willson, P., Harding, J. C., Bishop, S. C., Plastow, G. S., & Dekkers, J. C. | ||||
| (2016) | Genetic and genomic basis of antibody response to porcine reproductive and respiratory syndrome (PRRS) in gilts and sows. Genetics, selection, evolution | GSE. 48(1), 51 |

















