



Steps to mitigate cross-contamination of piglet processing fluids with PRRS MLV
As piglets move from the sow farm to the growing stage, it’s important to know their porcine reproductive and respiratory syndrome (PRRS) status.Typically, that has been achieved by collecting serum from a few dozen pigs. But as piglet processing fluids (PF) have gained traction, that option has expanded testing to include hundreds of pigs at a time. Certainly, PF are quicker and easier for farrowing-room caretakers to collect than serum.
One challenge that has surfaced is the potential cross-contamination of samples when a PRRS modified-live (MLV) vaccine is administered at processing. “Anyone who’s processed a pig or watched a team knows there are numerous ways that can happen,” said Daniel Brown, veterinary student at the University of Illinois. “For one, the tools are often placed in the same tote.”
Brown conducted a study to validate a method to mitigate such cross contamination, with the secondary objective of assessing possible risk factors.1
Three processing protocols
The study took place on a 6,500-head sow farm that, following a PRRS break, was vaccinating piglets with a PRRS-MLV at processing. For several weeks before the study began, numerous PF samples collected by the farm staff yielded positive results for PPRS virus by polymerase chain reaction (PCR). “Sequencing revealed 100 percent homology to the PPRS-MLV vaccine that the farm was using,” Brown told Pig Health Today.
Piglet processing was organized into three protocols:
- Control — No PRRS-MLV vaccine was administered at processing (30 litters).
- Standard — Followed the farm’s protocol where one technician was responsible for piglet processing (castration, tail docking, ear tattoo, iron supplement) and administering PRRS-MLV vaccine at the same time (310 litters).
- Modified — Divided the tasks so that one technician processed piglets and one technician administered the PRRS-MLV vaccine, only after castration was completed (320 litters).
The piglets’ tails and testicles were placed into a plastic bag that was stored in the same tote as the processing tools. Fluids pooled from 10 litters made up one experimental unit or sample that was tested for PRRS virus by PCR. Results were considered negative at cycle threshold values greater than 37.
Results and risks factors
Neither the control nor the modified protocols recorded any PRRS PCR-positive results. The standard protocol had 12.9 percent positive samples, but that did not result in a statistical difference between the two groups.
“Interestingly, if we had achieved the same 26.3 percent positive samples that the farm saw in the month and a half leading up to the study, we would have surpassed the statistical threshold,” Brown noted.
To assess possible risk factors, Brown evaluated samples collected through the farm’s standard protocol to determine if there were any correlations with positive-PRRS PRC results. He focused on the technician collecting the sample, the order of the processing steps and disinfection. “While these were not statistically analysed, I did find some interesting results,” he added.
For example, the order of vaccination influenced test results. (See accompanying table.) Technicians who vaccinated after processing piglets had 7.5 percent fewer positives than those vaccinating before processing. “This could be due to vaccine particles on the technicians’ hands,” Brown said.
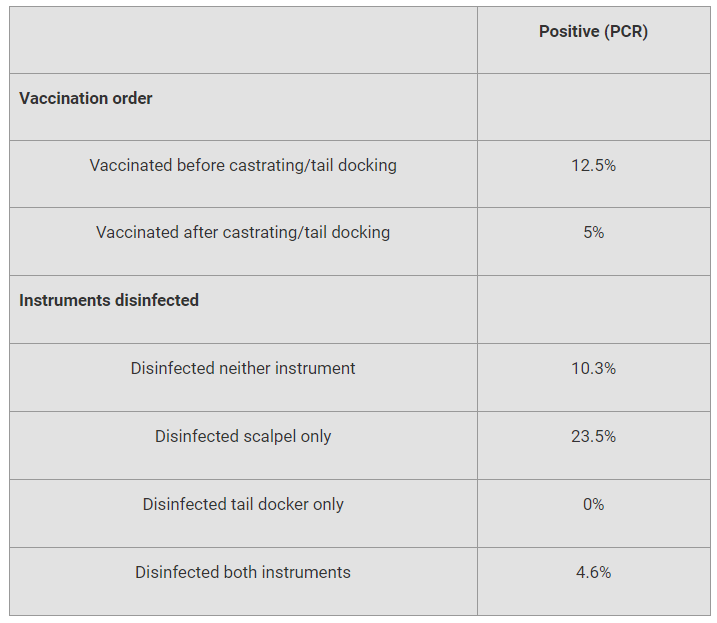
sup>* Risk factors assessed only for technicians using standard protocol.
Various combinations of disinfecting processing tools also were considered. Some of the variation in the disinfection results could be due to contamination of the solution. “This may be attributed to a technician using the same container of disinfectant to dip his instruments in throughout one day,” Brown added. Also, the farm used a chlorhexidine dip, which has been shown to be ineffective at reducing PRRS virus detection with PCR.
The technicians themselves also play a role. Of the 11 technicians following the standard protocol, six provided PRRS-positive samples and two of those yielded 50 percent of all positive results. “There could be numerous reasons for this,” he said, “from how the vaccines were handled to how thoroughly tools were disinfected.”
In conclusion
Although there was no statistically significant difference between the modified and standard protocols, Brown said the data does raise questions about the relationship between processing procedures and potential cross-contamination when vaccinating piglets with a PRRS-MLV vaccine.
“Moving forward, farms that rely on PRRS vaccination at piglet processing and PF for testing must consider the risk factors to avoid cross-contamination,” he concluded.
Assigning piglet processing and vaccination jobs to separate technicians is a good place to start. From a broader, industry view, Brown said additional research would be beneficial.
| References | ||||
|---|---|---|---|---|
| Brown D, et al. | ||||
| (2019) | Validation of a modified method of processing fluid collection to mitigate cross-contamination with a porcine reproductive and respiratory syndrome modified live virus vaccine.. Student Research, 50th American Association of Swine Veterinarians’ nnual Meeting. |








