Diagnostics at Work: Tissue Sampling
US - Tissue samples can be tested for the presence of a range of pathogens, according to Life Technologies.
For practical information on how to take tissue samples, view and download an informational brochure, “Guidelines for taking diagnostic samples from pigs: tissue,” courtesy of Thermo Fisher Scientific.
Diagnostic use
Detection of pathogens by microscopy—Some protozoa and several helminth parasites or their eggs, e.g., Ascaris suis, Balantidum coli, Strongyloides ransomi, Isospora suis, Oesophagostomum dentatum, Trichuris suis, as well as some viruses, e.g., rotaviruses, coronaviruses, etc., can be suspected or identified by using different microscopy techniques with tissue samples of the small and large intestine. Submission of other tissue samples to microscopy for the identification of pathogens is possible, but seldom performed.
Detection of pathogens by cultural testing—Tissue samples can be tested by culture for the presence of a range of bacterial respiratory, enteric, reproductive, and/or systemic pathogens. All bacteria that can be isolated from other samples, e.g., BALF, feces, nasal swabs, synovia, can also be isolated from the corresponding tissue.
Detection of pathogen RNA/DNA by PCR-based tests—The presence of pathogens can be confirmed in various tissues. However, the detection of several ubiquitous bacteria and viruses alone with highly sensitive PCR techniques does not confirm its relevance in clinical disease.
Animal selection
Deciding which animals to take samples from depends on the desired outcome:
Cause of death—Take tissue samples from pigs that died spontaneously. Be aware of autolysis, which precludes from examination pigs that have died more than 24 hours earlier.
Cause of disease—Take tissue samples from pigs that show the typical symptoms of the particular disease, preferably in the acute phase. Do not take samples from pigs showing stunted growth as the only sign, or from pigs showing additional signs of another disease that is of minor interest.
Sample size
In either case of spontaneous deaths or clinical disease at group level, a minimum of 3 pigs is recommended for post-mortem examination and subsequent tissue sampling. If symptoms are not consistent in the affected group, select at least 3 pigs per typical symptom; e.g., in a group with pigs showing either coughing or central nervous symptoms, select 3 pigs showing coughing as the main symptom and 3 pigs showing central nervous signs as the main symptoms.
Sample sizes may vary based on in-herd prevalence level of a disease, the tested disease itself, confidence level of the outcome, the requested test method, and the purpose of the sampling.
Preparation
When selecting living animals, separate these from the others before euthanizing them. Use electro-stunning whenever possible. If this is not the case, intravenous injection of anesthetizing drugs (e.g., ketamine plus azaperone), followed by an overdose of barbiturate, is most appropriate. Do not kill animals by head shot if further examination of brain tissue is the aim.
Do not take tissue samples in areas stocked with living animals.
Perform the necropsy in a clean area with concrete floor and enough light. This area should be cleaned and disinfected immediately after the necropsy and should not be accessible to other pigs.
Sampling technique
- Open the different body cavities separately; start with the abdominal cavity, followed by the thoracic cavity and ending with the skull cavity.
- Use a cleaned and disinfected knife (saw for the head). Clean and disinfect the knife between pigs.
- Take samples at the visible border between affected and unaffected tissue.
- Use a sterile scalpel blade for sectioning the tissue. The samples should be approx. 2 cm along each edge. If different organs are affected, use at least one new blade per organ system. When sampling gut tissue, ligate both ends of the anticipated sample before cutting.
- Use a sterile collection tube for each tissue sample (10–50 mL). Tubes must not contain any salts.
- Carefully withdraw scalpel blades after use. Do not put them into the sample tubes.
- Label each tube immediately with the animal ID (ear tag number) and the type of tissue using a waterproof marker. Write numbers and letters clearly according to good clinical practice (e.g., 5 not 5 ).
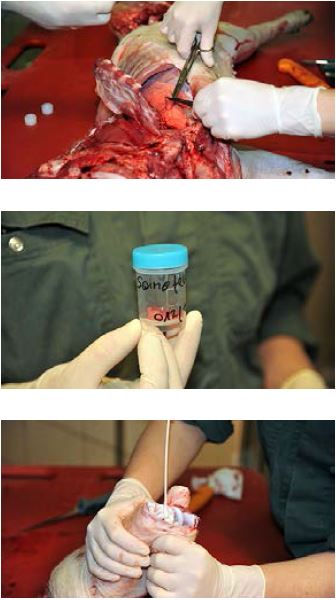
Storage
Store the sample in a refrigerator until shipment to the laboratory, which should be within 1 day.
Shipment
Material from diseased animals is usually classified as "Biological substance, category B" according to UN regulations (UN 3373). It must be shipped in compliance with national regulations and, at least for international shipment, in compliance with “Packing Instruction 650” specified by the International Air Transport Association (IATA). National regulations and IATA instructions may change over time. If you have doubts about the actual regulations, please ask your courier or the lab.
The sample should be accompanied by a case history and examination form, including:
- Name of veterinarian
- Name of farmer/herd owner
- Invoicing information
- Species/breed and age of sampled animals
- Date samples were taken
- Number of samples
- Type of samples
- Identification/labelling of samples (correlation between numbers on the samples and ear tags on pigs)
- Specified test that should be performed, such as “quantitative real-time PCR for Lawsonia intracellularis” rather than just “L.i. detection”; or “electron microscopy for rota and coronaviruses” rather than just “Rota/Corona”
- Results from any previous tests which do not need to be repeated
Good background information can help the laboratory conduct the most appropriate tests and provide advice in context.
This brochure is for informational purposes only. It is the responsibility of the customer to perform its own analysis and appropriate internal validation studies to ensure that the products and services it obtains from us satisfy or will satisfy the customer’s requirements and are fit for the customer’s animal health applications.
Further information
Please contact your local animal health testing laboratory or go to lifetechnologies.com/animalhealth