



Diagnostics at Work: Nasal Swabs
GLOBAL - Nasal swabs can be tested by culture for the presence of bacterial respiratory pathogens. For practical information on how to collect nasal swabs, view and download an informational brochure, “Guidelines for taking diagnostic samples from pigs: nasal swabs,“ courtesy of Thermo Fisher Scientific.
Detection of bacterial respiratory pathogens — Nasal swabs can be tested by culture for the presence of B. bronchiseptica and toxigenic P. multocida jointly causing progressive rhinitis atrophicans (pRA). A very careful interpretation is recommended when commensals of the upper respiratory tract, such as H. parasuis, M. hyorhinis, or S. suis, are found in nasal swabs; such findings cannot be used to determine the aetiology of any respiratory diseases.
Detection of viral respiratory pathogens and M. hyopneumoniae RNA/DNA (PCR-based tests) — The presence of pathogens that are difficult to cultivate can be confirmed in nasal swabs by PCR. Detection of SIV supports a presumptive diagnosis of influenza outbreak, whereas (no) detection of M. hyopneumoniae can be of interest in the context of monitoring SPF herds with suspicious clinical signs.
Animal selection
Deciding which animals to take samples from depends on the desired outcome:
Detection of infection—Select animals with clinical signs.
Absence of infection—Select animals with clinical signs; if there are no symptoms present, take samples from animals selected at random during a walk through the pens.
Tracking infection status over time (i.e., longitudinal examination)*—Take the first samples on day 1 and repeat samples from the same animals at appropriate time intervals.
To determine the infection status in different groups (i.e., cross-sectional examination)*—Take samples from animals of different ages, e.g., 4, 8, 12, 16, 20, and 24 weeks of age.
*If serological testing is to be used, send all samples to the laboratory in one batch to avoid potential variation between different batches of test kits.Sample size
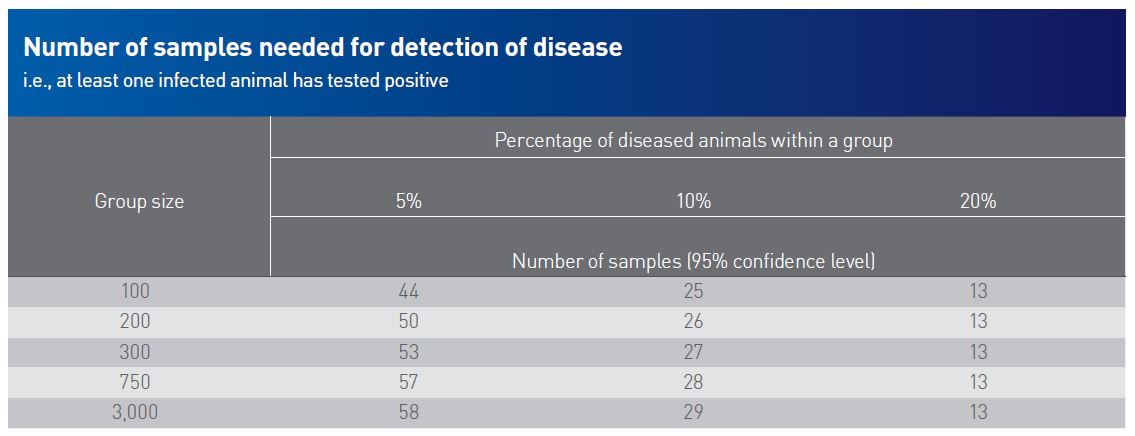
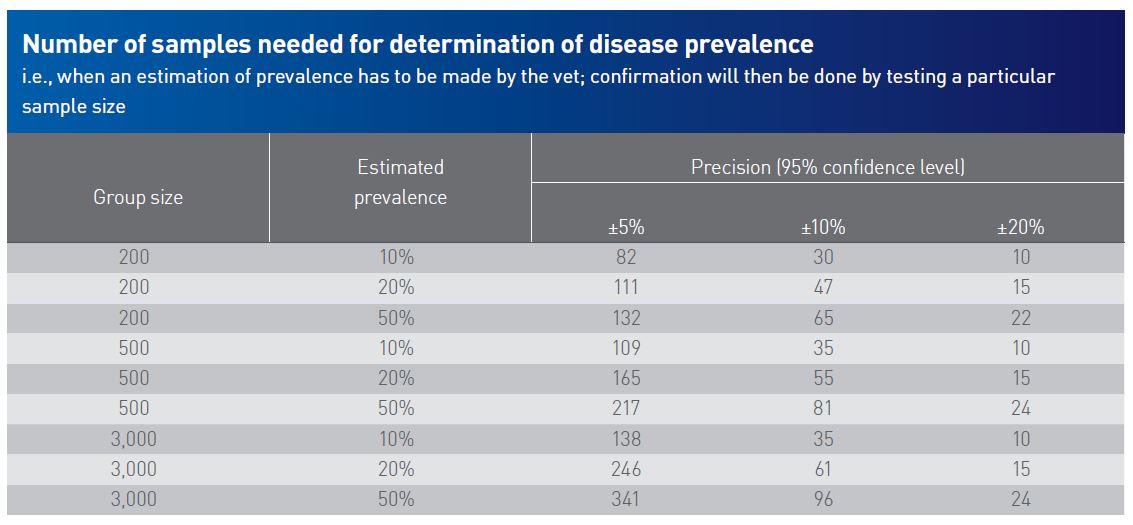
Sample size may vary based on in-herd prevalence level of a disease, the tested disease itself, confidence level of the outcome, the requested test method, and the purpose of the sampling.
Preparation
Do not take samples from animals in extensively overcrowded pens—pigs may panic and hurt each other or the veterinarian during sampling.
Make sure animals are properly restrained in an appropriate fashion by a competent person.

- Ensure there is enough light in the work area.
- Wear ear plugs or ear defenders.
- Use swabs of a diameter and length appropriate to the weight of the pig.
- The stem of the swab should be made of plastic, which is less likely to break than a wooden stem (pigs often make defensive movements during sampling).
- The tip of the swab should be flocked with a synthetic fibre (e.g., Dacron) rather than with cotton, if PCR is intended as the final diagnostic test. Cotton fibres do not release a sufficient amount of material swabbed from the nasal epithelium.
- For cultural testing: use swab containers with medium (e.g., Amies).
- For PCR testing: use swab containers without medium.
- If both methods are required, take two swabs from each pig and use the two different types of containers.
Sampling technique
- Restrain the pig. Use an iron snare if needed. Pay attention to the correct positioning of the snare, which should be always beyond the first premolars.
- Clean the nose with a dry piece of paper.
- Push the swab deep into the ventral passage of the nose and leave it in this position for 3 seconds; to avoid bleeding, do not injure the nasal conchae by applying too much pressure.
- Rotate the swab one third around the central axis and leave it again for 3 seconds.
- Again rotate the swab one third around the central axis and leave it for 3 seconds.
- Place the swab in the appropriate container.
- Label the container immediately with the animal ID (ear tag number) using a waterproof marker. Write numbers and letters clearly according to good clinical practice (e.g., 5 not 5).
NOTE: Steps 4 and 5 significantly increase the amount of material captured by the swab and thereby the diagnostic sensitivity of the whole method.
Storage
The sample should be stored in a refrigerator until shipment to the laboratory, which should be within 24–36 hours. If this is not possible and only PCR is required, freeze the sample at –20 to –80°C. Keep in mind that no further cultural examination is possible after freezing a sample.
Shipment
Material from diseased animals is usually classified as "Biological substance, category B" according to UN regulations (UN 3373). It must be shipped in compliance with national regulations and, at least for international shipment, in compliance with ”Packing Instruction 650” specified by the International Air Transport Association (IATA). National regulations and IATA instructions may change over time. If you have doubts about the actual regulations, please ask your courier or the lab.
The sample should be accompanied by a case history and examination form, including:
- Name of veterinarian
- Name of farmer/herd owner
- Invoicing information
- Species/breed and age of sampled animals
- Date samples were taken
- Number of samples
- Type of samples
- Identification/labelling of samples (correlation between numbers on the samples and ear tags on pigs)
- Specified test that should be performed, such as ”real-time PCR for M. hyopneumoniae” rather than just “M. hyo detection”
- Results from any previous tests which do not need to be repeated
Good background information can help the laboratory conduct the most appropriate
tests and provide advice in context.
This brochure is for informational purposes only. It is the responsibility of the customer to perform its own analysis and appropriate internal validation studies to ensure that the products and services it obtains from us satisfy or will satisfy the customer’s requirements and are fit for the customer’s animal health applications.
Further information
Please contact your local animal health testing laboratory or go to lifetechnologies.com/animalhealth.









