APHA Report: Porcine Circovirus, PRRS, Swine Influenza
UK - The Animal and Plant Health Agency (APHA) has reported incidents including sudden deaths due to porcine reproductive and respiratory syndrome and enteric disease, swine influenza and spine abcesses in its Pig Disease Surveillance Monthly Report, combined for January and February 2015.Reproductive disease
Stillbirths and poor viability piglets due to porcine reproductive and respiratory syndrome
Porcine reproductive and respiratory syndrome (PRRS) was diagnosed as the cause of stillbirths, birth of low viability piglets and increased preweaning mortality on an indoor breeding unit on which sows were vaccinated for PRRSv.
Sows of affected litters did not appear unwell. Eight full-term foetuses were submitted, two of which had uninflated lungs and were true stillbirths. Three were tested for PRRS and one was PCR-positive, confirming the diagnosis.
Enteric Disease
Sudden deaths due to enteric disease in neonatal piglets
Two incidents of enteric disease were diagnosed when piglets were submitted to investigate sudden deaths.
The first involved clostridial enterotoxaemia which was diagnosed in two pigs submitted to investigate disease in piglets ongoing over six farrowing batches on an outdoor breeding unit. If piglets were drenched with anticoccidial treatment at 3-5 days old there was no problem, but without treatment, diarrhoea was seen from day 12 to 28 with 95% of piglets affected in some batches.
The submitted pigs were found dead at 14-days-old and in the batch of 1800 piglets, five litters were affected with about 20 piglets found dead. There was some concern that this was an unseasonal emergence of Klebsiella species septicaemia which was diagnosed in this herd in August 2014, however both piglets were found to be dehydrated with severe necrotising enteritis.
The enteric lesions were typical of clostridial enterotoxaemia (type A or C) and this was supported by detection of Clostridium perfringens alpha toxin in the small intestine of one pig 2; and the presence of bacteria with clostridial morphology.
Histopathology also detected lesser involvement of Isospora suis coccidial infection. When clostridial disease occurs in older preweaned piglets, as found here, concurrent coccidiosis can be the predisposing factor and the clinical history supports this possibility in this case.
In the second incident, enteric colibacillosis was diagnosed in two-day-old piglets on an indoor breederfinisher. Five litters had been affected by sudden deaths of up to 20 piglets in total at one to two-daysold.
The small intestines of submitted piglets were dilated with watery pale fluid and the rectums were empty. Escherichia coli V17 was isolated from the intestines of both piglets and no other enteropathogens, including no porcine epidemic diarrhoea virus, were detected.
Diagnoses of rotaviral enteritis in neonatal pigs with diarrhoea
Rotavirus was detected in all of five faecal samples submitted from the Thirsk region to investigate diarrhoea and weight loss in three-day-old piglets and no other enteropathogen was identified.
Two diarrhoeic piglets were submitted to the University of Bristol to investigate malaise, diarrhoea and illthrift affecting almost all unweaned piglets on an 80-sow unit, with 12 piglets dying. The piglets had been kept in separate pens with their mothers but had nose to nose contact with other litters.
Watery pale yellow intestinal contents were found and laboratory testing confirmed the presence of rotavirus which is consistent with the high morbidity diarrhoea and illthrift with low mortality.
Interestingly, a monophasic Salmonella Typhimurium-like variant (4,5,12:i) was also isolated from the large intestinal contents although lesions typical of salmonellosis were not seen. This type of Salmonella has become the predominant serotype in GB pigs over recent years.
It is rarely implicated as the cause of diarrhoea in preweaned pigs but is one of the most common serotypes implicated in disease post-weaning.
At Shrewsbury, rotavirus was detected in a sample from an untreated piglet submitted to determine the cause of high morbidity diarrhoea in neonatal piglets on a 600-sow breeding farm. One thousand unweaned piglets were on the premises and approximately 80 piglets aged less than one week had a watery scour.
Enteric colibacillosis immediately after weaning
Enteric colibacillosis was diagnosed as the cause of diarrhoea in 25% of 900 six-week-old housed weaners with negligible mortality. Diarrhoea began two days after weaning and there was a poor response to treatment in water.
No Salmonella or porcine epidemic diarrhoea was detected but a profuse growth of haemolytic Escherichia coli strain V17 (O157: K’V17’) was isolated, these pig strains are not associated with human disease and are verocytotoxin negative.
The isolate did not test positive for the F4 adhesin (K88) and further serotyping was necessary to identify it as a pathogenic strain.
Pathogenic E. coli strains in pigs are not necessarily haemolytic and may be F4 adhesin negative and, where the clinical or pathological presentation is suspicious of E. coli involvement, full serotyping is recommended.
Interestingly, the isolate showed in vitro resistance to lincomycin/spectinomycin which had been used for treatment.
Salmonellosis with pilosicoli colitis in finishers with respiratory disease
Two intestinal samples were submitted from late finishers on an indoor 2000-pig nursery-finisher unit on which about 10% of 600 were affected with spreading respiratory disease, and later, diarrhoea.
Monophasic Salmonella 4, 12:i:- phage type 193 was isolated by direct culture from both samples, together with Brachyspira pilosicoli from one, pointing to salmonellosis, with spirochaetal colitis also contributing to the enteric disease.
It is not common to diagnose salmonellosis in older finishers and one can speculate that the intercurrent respiratory disease may have increased the pigs’ susceptibility to Salmonella, particularly if this involved immunosuppressive viruses (PRRSv or PCV2).
An alternative explanation is that antimicrobial treatment used for the respiratory disease altered the commensal intestinal flora in favour of Salmonella organisms, particularly as the Salmonella isolate was resistant to several antimicrobials used to treat respiratory disease.
Enteric disease due to porcine circovirus 2 with Brachyspira pilosicoli Watery-yellow diarrhoea and wasting was reported affecting about 50% of pigs in a group of 12-week-old pigs.
The indoor straw-based finisher herd affected received pigs from a nursery unit which itself filled from several sources. Some respiratory disease was also present in the pigs which were vaccinated earlier in rear on the nursery unit for Mycoplasma hyopneumoniae, PRRS and PCV2.
A batch of typically-affected pigs were euthanased and submitted to Bury St Edmunds for investigation. All had diarrhoea with a diffuse typhlocolitis. Brachyspira pilosicoli was detected by PCR and culture consistent with a diagnosis of spirochetal colitis.
However, significantly, histopathology and PCV2 immunohistochemistry on lymph nodes and intestines also confirmed enteric PCV2-associated (PCVAD) disease in these pigs, as well as spirochaetal disease.
The schedule involved PCV2 vaccination of pigs on the nursery unit supplying the farm two weeks after weaning. Whether it was this delay of vaccination into the post-weaning period, poor compliance with the vaccination schedule, or other factors which resulted in PCVAD occurring in vaccinated pigs, is being investigated.
There is evidence from those involved in PCV2 research that problems with PCVAD can occur due to variation in maternal antibody levels between piglets at the time that they are vaccinated.
Thus, while PCV2 vaccination two weeks after weaning may be suitable for some pig populations, if maternal antibody to PCV2 is low at weaning and there is significant challenge, the timing could be too late for others.
There is a webinar by Joaquim Segales on circovirus vaccination and the management of maternally-derived antibodies which can be accessed via this link: http://www.workcast.com/register?pak=8142213214869787.
This case illustrates the advantage of post-mortem examination and sampling in obtaining a full diagnosis rather than submitting just faecal samples.
In this case, PME was prompted by the high morbidity and degree of wasting.
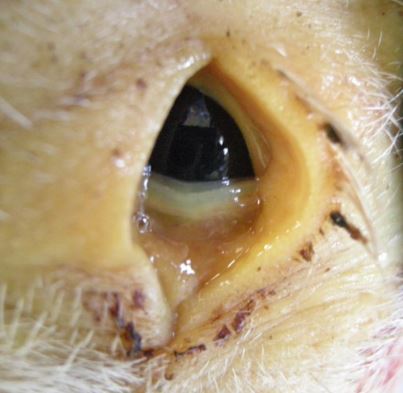
Jaundice due to porcine circovirus 2-associated disease
Mixed findings were present in three culled six-week-old pigs in poor body condition which were submitted to Bury St Edmunds to investigate a problem of wasting and diarrhoea.
Disease had been ongoing in successive batches for two to three months on the indoor breeder-finisher and, in the batch from which pigs were submitted, 70 of 220 pigs were affected and three had died.
One pig was jaundiced (Figure 1) due to porcine circovirus 2-associated hepatitis, a second had enteric colibacillosis and the third had a polyarthritis but no pathogens were isolated, probably because the pig had been treated.
Pigs were vaccinated for PCV2 at 20 days of age and the timing of vaccination merits reevaluation given the finding of PCV2-associated disease in one of the pigs, although PCVAD was not the sole cause of the clinical disease.
Respiratory Disease
Blue ears and coughing in pigs with porcine reproductive and respiratory syndrome
PRRS was diagnosed at Bury St Edmunds as the cause of coughing and cyanosis of the ears in a batch of 14-week-old pigs in which 20% were affected and 2% had died. Pigs were vaccinated for PRRSv, Mycoplasma hyopneumoniae and PCV2.
Three successive batches of pigs had been affected. Two pigs were submitted and both had pneumonias which affected most of both lungs in one pig and the cranial parts in the other, lymph nodes were also enlarged.
PRRSv was detected by PCR in the spleens and histopathology revealed severe bronchointerstitial pneumonias with lesions of the proliferative and necrotising pneumonia (PNP) of pigs which can be due to PRRS or combined PRRS and PCVAD.
PRRSv and PCV2 immunohistochemistry confirmed involvement of PRRS but not PCVAD in this case. The PRRSv strain was sequenced and found to be very similar to that detected in a February 2014 submission under the same ownership.
PRRS was also diagnosed at Penrith in a group of bought-in pigs of mixed ages which suddenly started to show signs of coughing, diarrhoea, and skin discoloration and erythema. Trueperella pyogenes was isolated from pneumonic lung and was likely to be secondary to the PRRS.
PRRS with disease due to Actinobacillus pleuropneumoniae
Twelve sudden deaths of pigs in good body condition were reported in a group of 360 14-week-old finishing pigs. There was some coughing in the group and the pigs had been treated with amoxicillin for three days with some response.
Two pigs were submitted to Thirsk and examination revealed severe pneumonias with large well-demarcated firm dark discoloured areas in all the lung lobes. Actinobacillus pleuropneumoniae was cultured from the affected lung tissue and PRRSv (Genotype 1, European type) was also detected in the pigs.
Three incidents involving infection with both APP and Streptococcus suis in finishers
There were two incidents of respiratory disease involving Actinobacillus pleuropneumoniae (APP) on different units.
The first of these was detected when APP and Streptococcus suis type 2 were both isolated from a swab collected from pneumonic lung during on-farm post-mortem examination of a pig which was one of eight to die over a seven-day period.
Ten per cent of the pigs on this indoor finishing unit were affected with respiratory disease at 18-weeks-old. As only a swab was submitted, it was not possible to determine whether there was concurrent viral disease involved.
The isolate showed in vitro sensitivity to ampicillin; to date just one A. pleuropneumoniae isolate from the East Anglian area has shown beta-lactam resistance.
In the second, a problem of pigs becoming pale and dying following transfer from straw yards to slats on a finisher unit was investigated.
There was a known issue of low-grade APP on the unit. Two typical pigs were submitted to Sutton Bonington and, in both, there was anaemia due to haemorrhage from severe chronic ulceration of the par oesophagea in the stomach.
Small intestinal contents were haemorrhagic and in the large intestine were dark brown and dry. Both pigs had significant pneumonias from which APP and Streptococcus suis 2 were isolated.
The presence of pneumonia is a recognised risk factor for gastric ulceration, possibly relating to inappetance.
In a third incident, three plucks were submitted from a large indoor nursery-finisher unit on which 10% of a group of 1000 pigs were showing respiratory disease and nine pigs had died in the three days prior to submission.
Pleuropneumonias were present with firm dark red areas of consolidation in dorsal lungs which were suggestive of APP infection and this organism was isolated.
APP was not the only pathogen identified; Pasteurella multocida and two different Streptococcus suis (type 2 and an untypeable strain) were also cultured although the lesions make it likely that the APP was the primary pathogen. No viral involvement was identified.
Swine influenza outbreaks in growing pigs
A pandemic H1N1 2009 swine influenza outbreak was diagnosed at Bury St Edmunds with Glässer’s disease in five-week-old pigs on an outdoor unit. The pigs were in a group of 500 which were coughing and wasting.
Three were submitted with varying degrees of pneumonia, pleurisy, pericarditis and arthritis. The patchy cranioventral pulmonary consolidation present in two pigs was suggestive of active swine influenza and this virus was detected by PCR in two pigs while Haemophilus parasuis was cultured from the pericardium of one pig.
Swine influenza was also the cause of respiratory disease affecting housed grower pigs at around 10 weeks of age. The disease was characterised by coughing and dyspnoea.
Three out of a group of 15 were affected at the time of submission and some adult pigs in the herd were also showing mild respiratory signs. A live dyspnoeic pig was submitted to Shrewsbury with mild cyanosis of the ears and snout.
Well demarcated consolidation of lung tissue with a cranioventral distribution was present and swine influenza infection was confirmed by PCR (not pandemic H1N1 2009). Antibiotics had recently been administered and no bacteria or mycoplasmas were detected.
Glässer’s disease and pigs with gastric ulceration
Another submission yielded Glasser’s disease as part of mixed, but significant findings, in six-week-old pigs in which eight deaths occurred over a weekend from the group of 2300 on an indoor rearing unit, followed by another 13 deaths overnight prompting submission of four dead pigs.
One was a likely runt. Two had died due to fatal haemorrhage from deep ulcers of the pars oesophagea and the fourth pig had a severe fibrinous polyserositis and pneumonia from which Haemophilus parasuis was isolated.
It is possible that the pigs with gastric ulcers also represented part of an H. parasuis outbreak and developed ulceration following inappetance due to acute H. parasuis infection although this was not cultured.
Lungworm in smallholder finisher pigs
Two out of a group of four pigs aged four months died having been listless for the previous three days.
On-farm post-mortem examination by the practitioner revealed diffuse areas of consolidation in the lungs by the practitioner and samples submitted to Shrewsbury confirmed the presence of lungworm identified as Metastrongylus apri.
Histopathology showed an eosinophilic bronchiolitis with several nematodes visible within the larger diameter airways. Earthworm are the intermediate host for this lungworm and can survive for years, allowing infection to persist on land and carry over between groups of pigs.
Systemic Disease
Outbreaks of streptococcal disease in post-weaned pigs
In one of these, an increase in mortality was reported in housed 14 to 17-week-old pigs with mortality rising to 8% over the previous two months with pigs being found dead unexpectedly.
Three hearts were received for examination and all had significant vegetative endocarditis lesions on the heart valves. Streptococcus suis type 2 was isolated from the lesions.
In the second, three seven-week old pigs were submitted to Shrewsbury following increased losses (>20 pigs) over the few days prior to submission from a batch of 3500 growing pigs.
The pigs had been sourced from four holdings as weaners. Some were found dead and others were seen to be lame.
Septicaemia, pneumonia and joint infections associated with Streptococcus suis type 2 were confirmed in the submitted pigs.
A third outbreak was diagnosed as the cause of significant mortality with pigs being found dead and others having swollen joints.
Forty five of 150 affected pigs had died from a group of 740 by the time they were seven-weeks-old on an indoor rearing unit. Three which died overnight were submitted and had non-specific gross lesions including reddening of the skin of the ventral body, excess fluid and fibrin stranding in body cavities and reddened lymph nodes, which were suggestive of septicaemias.
This was confirmed by the isolation of Streptococcus suis type 2 from several internal sites in all three pigs and the meninges of one.
Erysipelas in finishers in strawed yards
Ten of a group of 150 finishers aged 16-20 weeks died unexpectedly over a ten day period. The pigs were kept in strawed pens and losses occurred across several pens.
600 pigs had already gone to slaughter from the batch without any issues. Three carcases were submitted to Shrewsbury and all showed advanced fibrinous to fibrous pericarditis and florid mitral valve vegetative endocarditis.
Enlargement and mottling of the livers present in two pigs was likely to reflect right sided heart failure. Likely thromboembolic lesions visible as pale foci were also present in two pigs’ kidneys.
Bacteriology on the heart valve lesions confirmed the presence of Streptococcus dysgalactiae equisimilis and Erysipelothrix rhusiopathiae, the latter likely to be the more significant clinical isolate.
Nervous Diseases
Type A2 porcine congenital tremor
Two five-day-old Gloucester Old Spot piglets were submitted live to investigate severe head and body tremor and splaying of all four limbs. The piglets submitted were from the second affected litter, which had both been sired by a newly purchased boar.
Histopathology with special staining of the brain and spinal cord revealed pronounced hypomyelination, confirming a diagnosis of porcine congenital tremor (CT) type A2. This is caused by a transmissible agent, the identity of which is still not known.
Sows having affected piglets do not have affected piglets in subsequent litters, considered to be due to the development of immunity. Most piglets with type A2 CT recover by weaning.
Breeding gilts with bacterial meningitis
Bacterial meningitis was diagnosed at Bury St Edmunds in two three to four-month-old replacement breeding gilts from a group of 40 in which 12 were showing nervous signs and eight had died.
A week earlier, the pigs had escaped into woodland and there was concern that the deaths related to this. There was fibrin stranding in the pericardial sacs and peritoneal cavities, a cloudy appearance to the meninges on the ventral aspect of the brains and one pig had a fibrinopurulent polyarthritis.
Together these findings suggested a bacterial cause but no pathogens were isolated. Histopathology confirmed severe acute to subacute purulent meningitis consistent with a bacterial cause with Haemophilus parasuis or Streptococcus suis being the most likely to be involved.
Musculoskeletal Diseases
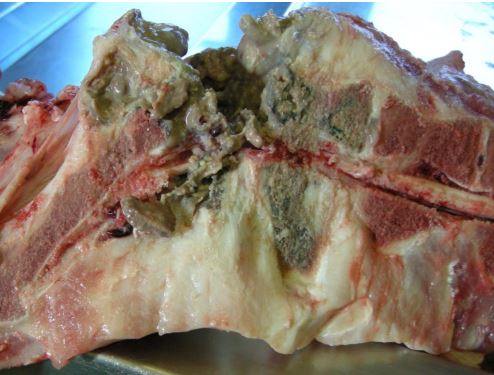
Spinal abscesses likely secondary to tail trauma
Severe suppurative and necrotising osteomyelitis at two sites in the spinal column and in the terminal tail vertebra was found to be the cause of recumbency of a replacement gilt, the second to be affected on an indoor breeding unit.
Figure 2 shows the lesions in the lumbosacral region and spinal column abscesses also affected thoracic vertebrae 6 and 7 where there was extension to an adjacent pleural abscess.
The vertebrae were disintegrating at affected sites. The distal 2 cm of the tail was black and necrosed.
Trueperella pyogenes and Fusobacterium necrophorum were both isolated and routine culture of the spinal abscesses for Brucella suis was negative. The origin of infection may have been tail trauma or haematogenous spread from an earlier systemic infection.