Legal requirements
Based on EU directives.
Medicines must be used safely and correctly in food producing animals to ensure there are no residues. Most countries across the world have strict controls over both the methods of prescribing and the uses of medicines. In the EU for example medicines may be considered in five groups, which are adapted in the UK as follows:.
- GSL: This describes the General Sales List and includes a variety of medicines that are available to the general public over the counter.
- P: This describes those medicines that are available over the counter only from a qualified Pharmacist where his expert advice and guidance can be given or supplied by a veterinarian for animals under his care.
- PML: Into this category are designated over the counter Pharmacy Merchant Lists. Included in this group are recognised and certified merchants who are able to sell from a prescribed list of medicines direct to the farming community.
- POM: These are Prescription Only Medicines that are only available on the direction of a veterinarian.
- Controlled Medicines: This category covers the addictive medicines such as morphine, heroin and pethidine.
The major categories concerning the pig farmer are the P, PML and POMs.
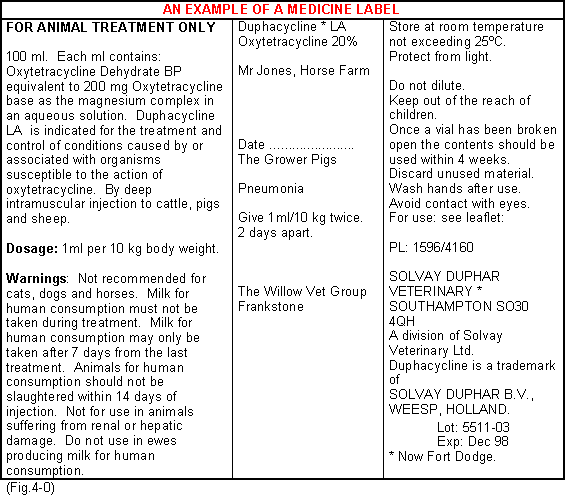
When a medicine is supplied to the farm the following information should be available:-
- A description of the medicine.
- The date of manufacture.
- The date of dispensing.
- The date of expiry.
- The client's name and address.
- The species to be treated.
- The date of withdrawal.
- The dose rate and instructions for use.
- Name and address of the supplier.
- Manufacturer's batch No.
- The name and address of the veterinarian prescribing.
A typical bottle label would appear as shown. (Fig.4-0)