



The path to genetically healthier pigs
Pathogens are a global threat to the swine industry. They arise from bacterial, viral, fungal, and parasitic sources. Numerous and often prone to mutation, with a tendency to become immune to treatment efforts over time.Production flows within the North American swine industry routinely face challenges from multiple pathogens, and a resultant annual cost to the North American swine industry estimated in the billion-dollar range; with PRRS estimated at $664 million alone (AASV 2011 Position Statement on PRRS elimination). This suggests the swine industry would benefit from animals that have a higher capacity to survive health challenges.
An animal’s ability to withstand a disease challenge can be defined in many ways. Resistance, Resilience, Tolerance, and Robustness are all relevant descriptors. Genetic selection for each concept is uniquely defined, distinctively implemented, and depending on the circumstances, it can be beneficial under differing situations. Genetic selection for disease resistance seems optimal, as an animal resistant to a disease would appear to have an advantage over an animal that is not able to control a disease challenge; however, targeting a specific pathogen requires a considerable amount of upfront investment. Adding to that, the earlier-mentioned fact that there are numerous pathogens presents a real challenge to become resistant to them all. Viruses also tend to mutate, which can lead to low-rewarding efforts should the targeted pathogen suddenly transform and become re-infective to the population.
Selection for disease tolerance instead of resistance recognizes that an animal can still perform under the burden of varying disease levels. However, selection for disease tolerance requires simultaneous measurements on both performance and the levels of infection. Another downside to genetic selection for resistance or tolerance is the challenge of acquiring continuous records of the pathogen burden. The burden levels will likely vary over time, yet are required for genetic selection in both instances (Doeschl-Wilson et al., 2012).
A final hurdle is that pathogen burdens do not exist within high-health nucleus herd environments that the pork industry demands. Therefore, an alternative approach is warranted.
The concept of disease resilience offers the aforementioned alternative. It is a combination of resistance and tolerance and is defined as the ability of an animal to maintain performance across environments when exposed to pathogen challenges (Albers et al., 1987). Disease resilience is unique in that selection in favor of does not require specific knowledge of any certain pathogen or level of challenge. The resilience phenotype can, therefore, be considered robust across stressors, both health-related and non-health-related, and more practical for selection on the population’s future fitness when considering there will be new pathogens that arise for which we are currently unaware.
Selection for disease resilience targets those genes that allow an animal to have a tolerating consequence, or quicker recovery in their performance when challenged (Figure 1). Instead of targeted gene discovery for a single pathogen, the selection is placed on a multigenic scale within the genome, using quantitative and molecular tools inside our genetic toolkit. The Genesus answer to healthier pigs lies within genetic selection for disease resilience phenotypes.
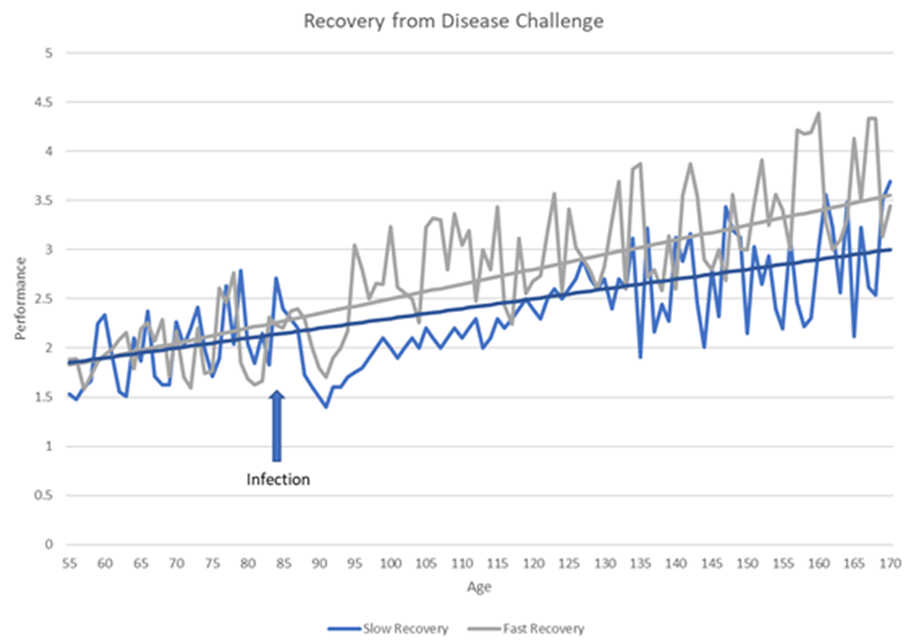
Genesus has been involved in funding disease research for over 10 years. Several useful tools have been discovered from genome-wide association analyses and incorporated into the Genesus toolkit.
- We have uncovered genomic regions that we place under selection to improve resilience to Porcine Reproductive and Respiratory Syndrome Virus (PRRSV).
- We are monitoring genomic regions found to affect susceptibility to Porcine Circovirus Associated Disease (PCVAD).
- Genesus is staying abreast of outside research and benefiting from our relationships within academia to improve our population’s resilience to E.Coli challenges.
Furthermore, disease resilience traits have more recently been identified, which will aid in filling the previously empty, phenotype void. These phenotypes are necessary for selection purposes, and Genesus is now able to key in on those specific attributes to identify more disease resilient animals.
The trek is not finished, however. Active research continues, and more tools continue to unfold for use in genetic selection for improved health. Diseases are becoming more numerous and geographically spread. For this reason, selection for disease resilience must continue to be a key component inside the genetic selection programs of swine.
Genesus continues its involvement in disease research and is working to actively combine genomics and disease resilience phenotypes in the selection for higher-health animals. In the forthcoming months, we will share more of the breakthrough discoveries from our involvement in major research projects involving Genome Canada, Genome Alberta, PigGen Canada, USDA National Institute of Food and Agriculture (NIFA) and the Alberta Meat and Livestock Agency. Through Genesus and these funding agencies, collaboration with projects and researchers at several major Universities around the world (e.g. University of Alberta, University of Saskatchewan, University of Guelph, Iowa State University, Kansas State University and University of Edinburgh) has transpired over the past decade.
We look forward to sharing in more detail what implementation has occurred inside of Genesus from our collaboration with researchers at these organizations, and our involvement in utilizing these resources.
| References | ||||
|---|---|---|---|---|
| Albers, G. A. A., G. D. Gray, L. R. Piper, J. S. F. Barker, L. F. Lejambre, and I. A. Barger. | ||||
| (1987) | The genetics of resistance and resilience to Haemonchus contortus infection in young Merino sheep.. Int. J. Parasitol. | 17:1355–1363. | ||
| Doeschl-Wilson, A. B., B. Villanueva, and I. Kyriazakis. | ||||
| (2012) | The first step towards genetic selection for host tolerance to infectious pathogens: Obtaining the tolerance phenotype through group estimates.. Front. Genet. | 3:265. |







