Research: Complete Workflow Solution for SIV Testing
GLOBAL - Swine influenza virus (SIV) is a highly contagious viral infection of pigs, resulting in significant economic losses in the swine industry and posing a significant threat to human health through zoonotic transmission. This study indicates that the SIV testing workflow, consisting of nucleic acid purification and USDA-licensed screening and subtyping tests, provides an economical and rapid solution for SIV screening and subtype identification to help control the disease. SIV subtypes are defined by the surface glycoproteins: hemagglutinin and neuraminidase, with H1N1, H3N2, and H1N2 representing the predominant subtypes in swine.
SIV subtypes are defined by the surface glycoproteins: hemagglutinin and neuraminidase, with H1N1, H3N2, and H1N2 representing the predominant subtypes in swine.
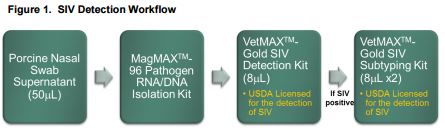
We have validated an SIV testing workflow consisting of high throughput nucleic acid purification, SIV detection, and SIV subtyping from porcine nasal swab samples (Figure 1). SIV can be detected using the USDA-licensed VetMAX™-Gold SIV Detection Kit, a single tube one-step real-time RT-PCR kit for the rapid and accurate screening for influenza A.
The assay targets three independent regions of the SIV genome to dramatically limit the number of false-negatives due to mutation of the viral genome. The VetMAX-Gold SIV Detection assay also incorporates multiple degenerate primers and probes designed to detect all known strains of SIV. It is multiplexed with an internal positive control (IPC) to monitor for nucleic acid recovery and PCR inhibition. Laboratories wishing to obtain more information about SIV-positive samples can utilize the VetMAX™-Gold SIV Subtyping Kit to further characterize their samples and confirm positive results. The VetMAX-Gold SIV Subtyping Kit is a pair of single-well real-time RT-PCR assays to detect and differentiate the H1, H3, N1 and N2 alleles.
We validated the screening and workflow by testing >100 SIV-positive and >100 SIV negative porcine nasal swab field samples and virus isolates originating from diverse geographic regions in the US with the screening and subtyping kits. The SIV status and subtype of each sample was confirmed prior to the start of the study with Virus Isolation (VI) and/or whole genome sequencing.
Collaborator laboratories purified the viral nucleic acid using the MagMAX™-96 Viral RNA Isolation Kit (AM1836) and MagMAX Express-96 Magnetic Particle Processor. Extracted nucleic acid (8µL) was tested with the VetMAX-Gold SIV Detection Kit and VetMAX Gold SIV Subtyping Kit on the Applied Biosystems™ 7500-Fast Real-Time PCR System.
Results of validation testing were used to determine diagnostic sensitivity and specificity for each kit. Detection with the VetMAX-Gold SIV Detection Kit resulted in calculated diagnostic sensitivity and specificity values of 98.4% and 99.1%, respectively. The VetMAX Gold SIV Subtyping Kit produced >97% sensitivity and specificity for identifying the SIV subtype from nasal swab samples. This study indicates that RNA isolated from diagnostic porcine nasal swab samples, tested with the VetMAX-Gold SIV Subtyping Kit in conjunction with the VetMAX-Gold SIV Detection Kit, provides an economical and rapid solution for SIV screening and subtype identification.
For more information about swine diagnostics, click here or connect to the Thermo Fisher Scientific Swine Resource Center.