



Prebiotics and probiotics boost pig growth and health
Dr Fausto Solis de los Santos discusses the use of prebiotics and probiotics in enhancing the gastrointestinal tracts of pigs to encourage more efficient feed conversion.Abstract
The gastrointestinal tract (GIT) of pigs is a very dynamic organ that interacts with the nervous, circulatory, endocrine and immune systems; therefore, the functioning of the GIT affects the entire physiology, health and well-being of an animal. Important intestinal features such as the villi, crypt, lamina propria and the intestinal microflora affect nutrient digestion and absorption, immunity, pathogen control and performance. With legal limitations on the use of antibiotic growth promoters, the industry and academia are testing several antibiotic alternatives, including prebiotics and probiotics, to maintain or improve feed efficiency in swine production. Prebiotics, such as Mannan-oligosacaharides (MOS), Fruto-oligosacharides (FOS), Galacto-oligosacharides (GOS), Chito-Oligosacharide (COS), Isomalto-oligosaccharides (IMO), Pectic-oligosaccharides (POS), and Xylo-oligosaccharides (XOS), are non-digestible feed ingredients that are fermented in the lower gut to select for beneficial bacteria. Probiotics refer to a group of non-pathogenic organisms that, when administered in sufficient numbers, are known to have beneficial effects on the health of the host animal; the most common probiotics are Bacillus (Gram-positive spore-forming bacteria), lactic acid-producing bacteria (Lactobacillus, Bifidobacterium, Enterococcus), and yeast. Both prebiotics and probiotics have shown to reduce pathogens, the former by offering receptors for the binding of pathogens like Salmonella. Probiotics have several mechanisms of action such as acidifying intestinal content; attachment to the intestinal epithelial surfaces to prevent pathogen attachment; competing for nutrients with pathogens; production of bacteria- toxins, and production of inhibitory substances, such as organic acids and hydrogen peroxide. Probiotics also stimulate specific and nonspecific immune entities such as IL and IgA. In swine nutrition, prebiotics and probiotics have shown to reduce diarrhoeal incidence, stimulate the immune system, reduce pathogenic bacteria and increase feed efficiency by an average of six percent. In conclusion, based on the review of several scientific literatures and the author experience, it is recommended to use prebiotics and probiotics in swine feed.
Full article
The gastrointestinal tract (GIT) is a very dynamic organ that interacts with the nervous, circulatory, endocrine and immune systems. The functioning of the GIT affects the entire physiology, health and wellbeing of an animal; two components, in particular, should be considered when enhancing GIT health: the intestinal mucosal membrane and the indigenous microflora.
The intestinal mucosal membrane consists of villi – finger-like structures with enterocytes for nutrient absorption (Figure 1) – and the lamina propria in the base of the villus, consisting of the peyer’s patch which monitors intestinal bacteria populations and prevents growth of pathogens. When a bacterium crosses the epithelial membrane, the dendritic cells stimulate the macrophages, which engulf the pathogen; this process also stimulates the maturation of antibodies, especially IgA. Intestinal villi also contain the crypt which produces goblet cells that are responsible for the production of mucous.
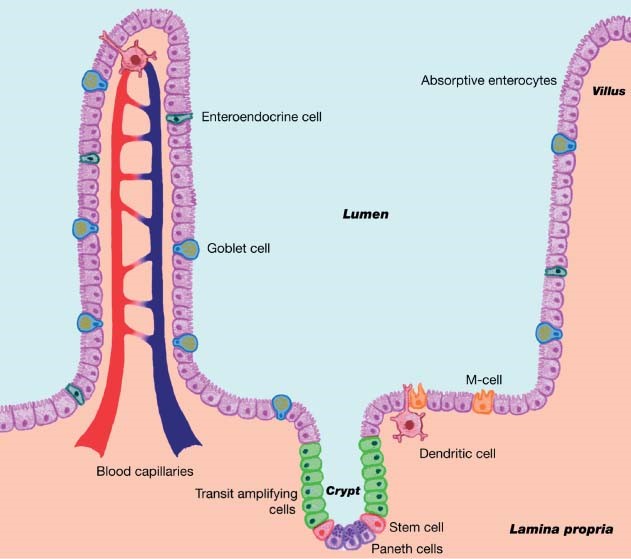
The mucous layer provides a protective blanket with sticky properties to entrap incoming bacteria. The mucous also maintains the indigenous microflora to provide protection against attachment of pathogens; also, in the porcine small intestine, goblet cells produce neutral mucin that contain sialic acid which is involved in interactions with bacteria.
The pig intestinal microflora is estimated to have more than 400 species of bacteria with a concentration 109 cfu/g of intestinal content, half of the bacteria are beneficial to the host – especially Lactobacillus and bifidobacterial – while the other half are pathogenic. For the impact of the GIT in animal health and performance, designing feeding programs and nutritional formulation to enhance the GIT membrane structures and stabilise microflora should be a priority for any nutritionist.
Since antibiotic residues may pass and accumulate in animal tissues that are then consumed by humans (Silfrany et al., 2012), bacteria may develop resistance to antimicrobials; therefore, alternatives such as plant extracts, bacteriocins, organic acids, enzymes, bacteriophages, prebiotics, and probiotics have been evaluated to stimulate intestinal health and performance in pigs.
Prebiotics enhance gut health in pigs
Prebiotics are non-digestible feed ingredients that are fermented in the lower gut to select for beneficial bacteria; during the fermentation, there is production of Volatile Fatty Acids (VFAs) mostly acetic, propionic and butyric acid that reduce the intestinal pH. It has been proven that pathogenic bacteria such as Salmonella, E. coli and Clostridium do not reproduce well in acidic environments. In addition, butyric acid stimulates the proliferation and differentiation of epithelial cells in the GIT, thereby, increasing the surface area for nutrients absorption (Pierce et al., 2007). VFAs serve also as sources of energy for animals.
There are different types of prebiotics such as Mannan-oligosacaharides (MOS), Fruto-oligosacharides (FOS), Galacto-oligosacharides (GOS), Chito-Oligosacharide (COS), Isomalto-oligosaccharides (IMO), Pectic-oligosaccharides (POS), and Xylo-oligosaccharides (XOS).
Prebiotics have immunomodulatory properties and the potential to reduce inflammation when supplemented into pig diets. At weaning, piglets face many stressors, such as change of environment, fight for hierarchy, and change from liquid to dry feed. These stressors cause intestinal villus atrophy, enzyme change, and higher production of Tumor Necrosis Factor-α (TNF- α), IL-1β, and IL-6, which are pro-inflammatory cytokines in the small intestine of pigs. The higher production of these pro-inflammatory cells reduces feed intake and increases incidence of diarrhoea. Lower feed intake and increased incidence of diarrhoea reduces body weight gain in the early post-weaning stage of pigs. Since enteric lactic acid-producing bacteria are reduced immediately in the post-weaning period, this may facilitate the colonisation of pathogens such as Clostridium spp, E. coli, and Salmonella.
One of the hypothesised mechanisms of action of growth promoters, antibiotics or feed additives is the reduction of inflammation (Niewold, 2007). The fact that the prebiotic supplementation is able to reduce inflammation may explain the improved animal performance observed when using them (Casey et al., 2004) (Table 1).
Table 1: the benefits of using prebiotics and probiotics in pig diets .jpg)
Inulin is a FOS prebiotic derived from Chicory that has showed to increase Lactobacillus and Bifidobacterium (Tran et al., 2016), and stabilise the health status of newly weaned piglets. Similarly, GOS-based prebiotics supplemented in the feed of growing-finishing pigs significantly increased Lactobacillus spp. (McDonnell et al., 2016). High population of Lactobacillus spp. is associated with reduction of S. Typhimurium numbers (Casey et al., 2004).
In several studies, prebiotics have shown to reduce the load of bacteria in the gut of swine (McDonnell et al., 2016) and improve resistance to bacterial colonisation, and also enhance the intestinal barrier function against invading pathogens (Table 1).
Probiotics are beneficial organisms that benefit pigs
Probiotics refer to a group of non-pathogenic organisms that when administered in sufficient numbers are known to have beneficial effects on health of the host animal.
The hypothesised mechanisms of action of probiotics are: 1) reducing the pH, which is an unfriendly environment for intestinal pathogens; 2) attachment on the intestinal epithelial surfaces to prevent pathogen attachment; 3) competition for nutrients with pathogens; 4) production of inhibitory substances such as organic acids, hydrogen peroxide, and bacteriocins; and 5) stimulation of specific and nonspecific immunity such as IL and IgA. Commercial probiotics could be divided into three categories: Bacillus (Gram-positive spore forming bacteria), lactic acid – producing bacteria (Lactobacillus, Bifidobacterium, Enterococcus), and yeast (NRC, 2012).
Since Lactobacillus-based probiotics grow very well in the gastrointestinal tract; they may confer enough protection for the entire growing period. However, these types of probiotics do not withstand high temperature environments such as pellet feeds. They can be used efficiently in mash feed and/or in pellet feed with a Post Pelleting Liquid Application (PPLA) system. Bacillus subtilis probiotics, on the other hand, resist temperature of up to 205 degrees F making this the probiotic of choice for pellet feed. The downside of Bacillus subtilis probiotics is the poor growth rate in the gut, which requires their supplementation throughout the entire grow-out period.
The effectiveness of probiotics is usually measured by the increase of the beneficial bacteria population number in the GIT. The Lactobacillus population increased from 7.39 log10 cfu/g to 7.49 log10 cfu/g of intestinal content when Bacillus subtilis probiotic was introduced into pig feed during a study conducted by Upadhaya et al. (2016). It is hypothesised that probiotic bacteria consume the oxygen in the gut leaving an anaerobic and unfriendly environment for pathogenic bacteria.
It has been shown that supplementing live yeast probiotics (Saccharomyces cerevisiae ssp. boulardii) to weaned pigs for 3 to 4 weeks increased villus height, epithelial cell proliferation, and the number of macrophages at various sites of the small intestine (Baker et al., 2013). These factors contributed to the observed improved post-weaning growth performance. Supplementation of probiotics in sow diets appears to reduce pathogen load and establish, more rapidly, the beneficial bacterial species, such as Lactobacillus, in the postnatal piglet, to defend against disease challenges and stimulate immune development (Baker et al., 2013). Probiotics have also proven to reduce the Inflammatory cells TNF, IL-10 (Table 1). Bifidobacterium-based probiotics also have remarkable effects against Salmonella and enterotoxigenic Escherichia coli (ETEC K88) in weanling pigs (Barba-Vidal et al., 2017).
Oral administration of the probiotic Streptococcus faecium to gnotobiotic piglets, challenged with various pathogenic strains of E. coli, resulted in increased weight gain and reduced severity of diarrhoea by reducing colonisation by pathogenic bacteria in the gut. Feeding the beneficial bacteria, Bifidobacterium lactis, reduced the concentrations of faecal rotavirus and E. coli, as well as decreasing severity of diarrhoea in piglets (Shu et al., 2001). Similar results were observed when feeding piglets diets containing Bacillus toyoi or Bacillus licheniformis and live yeast Saccharomyces cerevisiae ssp. Boulardii, where the incidence and severity of diarrhoea, number of enterococci and coliforms, especially ETEC in the intestines, decreased (Kyriakis et al., 1999). Probiotics have shown to improve feed intake in pigs in different stages, for example, a Bifidobacterium probiotic was shown to increase the feed intake in salmonella-challenged pigs (Emili-Barba-Vidal et al., 2017).
Finally, the use of multi-species probiotics has shown increased benefits over mono-species probiotics. This is due to the fact that probiotic effects are genera, species, and strain specific and having multiple species may improve the spectrum of the product.
By Dr Fausto Solis de los Santos

The author has a Master of Science and PhD degrees in Animal Nutrition.
References
Adami, et al., 1999. J. Basic Microbiol. 39:3–9.
Baker et al., 2013. J. Anim. Sci. 91:3390–3399
Barba Vidal et al.,2017. Front. Microbiol.,
Bontempo et al., 2006. Anim. Feed Sci. Technol. 129:224–236.
Casey et al 2004. Lett. Appl. Microbiol. 39:431–438.
Herfel et al., 2013. J. Anim. Sci. 91:907–913
Kyriakis et al., 1999. Res. Vet. Sci. 67:223–228.
McDonnell et al., 2016. J. Anim. Sci. 2016.94:153–156
Niewold. T. A. 2007. Poultry Science, Volume 86, Issue 4, Pages 605–609,
NRC. 2012. Nutrient requirements of swine. 12th ed.
Pierce et al., 2007. Anim. Feed Sci. Technol. 132:267−282.
Searle et al., 2009. J. Med. Microbiol. 58, 37–48
Silfrani, et al., 2012. Journal of Food Protection 2(2) 352-354.
Shu et al., J. Pediatr. Gastroenterol. Nutr. 33:171–177.
Stein et al., 2006. Anim. Biotechnol. 17:217–231
Tran, H et al., J. Anim. Sci. 2012.90:3049–3059
Upadhaya et al., 2016. J. of Anim. Sci., 94: Issue suppl_2, 1 Pages 81.
Underdahl et al., 1983. Prog. Food Nutr. Sci. 7:5–12.
Yang et al., 2012. J. Anim. Sci. .90:2671–2676







