



Managing Lawsonia and Brachyspira Infections Using Pharmacokinetic and Pharmacodynamic Principles
An understanding of these principles can help veterinarians decide which drug to use and at what dose to achieve the optimum results to control swine dysentery and ileitis, Dr David Burch of Octagon Services in the UK told the 2013 AASV Annual Meeting in paper written with J. Mark Hammer of Novartis Animal Health Inc.Introduction
To make informed decisions on which antibiotic to use and which route and at what inclusion/dosage level, it is important for practitioners to be familiar with the basic pharmacokinetic (PK) information for a drug and be able
to relate it to the pharmacodynamic (PD) information that is available.
Frequently, the minimum inhibitory concentration (MIC) is determined for Brachyspira hyodysenteriae, the cause of swine dysentery, as part of the diagnostic work up, as the usual PD information and this can be correlated with the PK or concentrations of antibiotic found in the colon contents. Similarly, for Lawsonia intracellularis infections, the cause of porcine proliferative enteropathy ('ileitis'), the ileum contents concentration is the major PK information but it is very difficult to grow L. intracellularis in cell cultures, so there is generally very limited intracellular MIC (iMIC) data available from field investigations.
The purpose of this paper is to correlate the PK and PD information for tiamulin (Denagard – Novartis Animal Health Inc.) against these two infections so that the practitioner can utilise his field information to make more informed treatment decisions regarding these primary enteric pathogens.
Pharmacokinetics of Tiamulin in the Gut
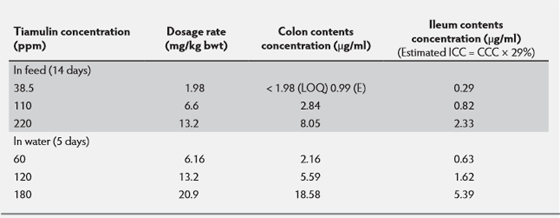
The concentrations that were achieved in the colon contents using a microbiological method, following administration of tiamulin in the feed at 38.5, 110 and 220ppm for 14 days are summarised in Table 1. It also looked at tiamulin's colon contents1 concentration (CCC) after giving it in the drinking water at 60, 120 and 180ppm for five days (see Table 1). Drugs accumulate in the colon contents and that the ileum contents concentration (ICC) was shown to be approximately 29 per cent of the CCC.3
These concentrations are helpful in giving an idea of the tiamulin-like bioactive concentrations that can be achieved in the certain sections of the gut. There may be some binding of the drug to the gut contents but this has not been taken into account. Tiamulin is only a moderate serum protein binder in pigs at about 30 per cent. Dose for dose, in feed medication achieved higher concentrations in the gut than water medication, possibly due to feed impairing the absorption and bioavailability of tiamulin.
Pharmacodynamics
Lawsonia intracellularis
Recent pharmacodynamic data for L.intracellularis1,2 really pushed forward our knowledge and understanding of how drugs worked against this infectious agent. Early pioneering work5,6 gave us some indication of what antibiotics were likely to work against L.intracellularis but they used a limited number of isolates and limited drug concentrations. A wider drug concentration range12 was used to test 10 US and EU isolates, to determine their iMICs and extracellular MICs (eMICs) and the determination was repeated (see Table 2). It was demonstrated3 that the iMICs were probably more significant when it came to determining PK/PD relationships, as L.intracellularis penetrates epithelial cells rapidly, within hours, leaving little time for exposure to an antibiotic and the iMIC determination more simulated the exposure to infected epithelial cells in the gut by antibiotics given in the feed and water.
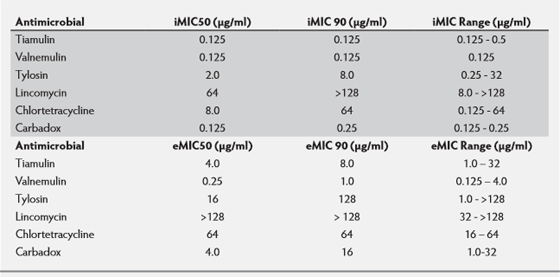
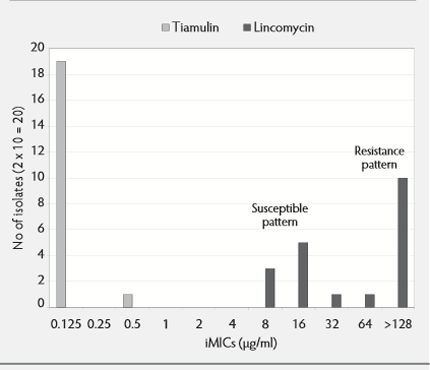
In addition different susceptibility patterns could be demonstrated for different antibiotics, which suggested that reduced susceptibility or even resistance development may have evolved in some cases, e.g. tetracycline and lincomycin but not to tiamulin, valnemulin, carbadox and tylosin.
Brachyspira hyodysenteriae
Interest in B.hyodysenteriae waned in the US over the last decade but has recently been rekindled with a number of outbreaks of Brachyspira spp associated diarrhoeas4 in North America. Europe has been struggling with the disease and resistance issues, especially since 2006 when certain growth promoters were banned, which also inhibited B.hyodysenteriae .
One of the first major reports5 described the MICs of 76 Australian isolates of B. hyodysenteriae, using a new microbroth dilution method (see Table 3).

From this data, sufficient information was available to formulate susceptibility patterns for the different antibiotics (see Figure 2).
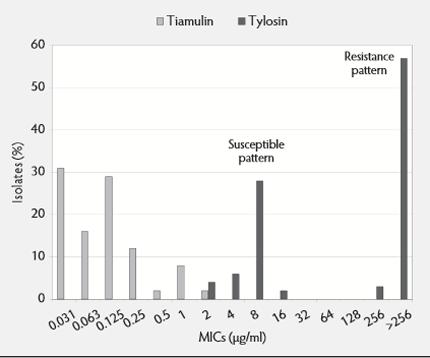
Tylosin showed a single-step resistance pattern, with 16µg/ml being the 'wild type' cut off or break-point, whereas tiamulin shows a two-step mutation pattern normally, with a 'wild type' cut off at 0.5µ/ml and then a first stage mutation to 2.0µg/ml. In Europe, strains have been found with MICs of more than 4.0µg/ml (a second mutation), which is considered the resistance break-point for tiamulin.
Integrating PK with PD
Lawsonia intracellularis
Using a simple approach the concentration of the drug should be above the MIC of the organism for sufficient time to either inhibit its growth or to kill the organism. The effect the drug has on the bug is dependent on the type of antibiotic, whether it is bacteriostatic or bactericidal and the organism itself. In the case of L.intracellularis, the drug needs also to penetrate the cell to exert its antibiotic effect.
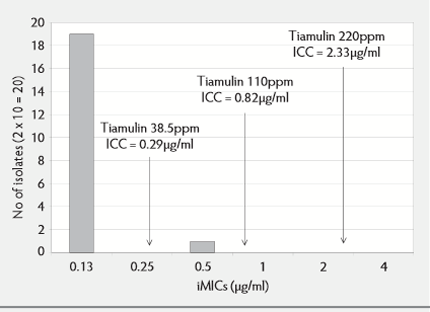
Tiamulin at 38.5ppm would be expected to exert an inhibitory effect (see Figure 3) against the majority of L.intracellularis isolates. The iMIC and intracellular minimum bactericidal concentration (iMBC) have not been determined so the iMBC: iMIC ratio has not been established. However, a trial11 showed there was an inhibitory effect when given in the feed at 38.5ppm for 28 days to pigs already infected with L.intracellularis, with an MIC of 0.25µg/ml.
Earlier work8 showed that 50ppm tiamulin in feed given prior to infection completely prevented the development of lesions caused by a strain of L.intracellularis with an iMIC of 0.125µg/ml. Tiamulin at 150ppm administered to pigs seven days after infection for 14 days completely eliminated the infection, demonstrating a more bactericidal, eliminatory effect8 at ICC concentrations 13 times the iMIC.
Brachyspira hyodysenteriae
With regard to B.hyodysenteriae, the MIC susceptibility pattern can be compared with the CCCs achieved with tiamulin given in feed and in water (see Figure 4).
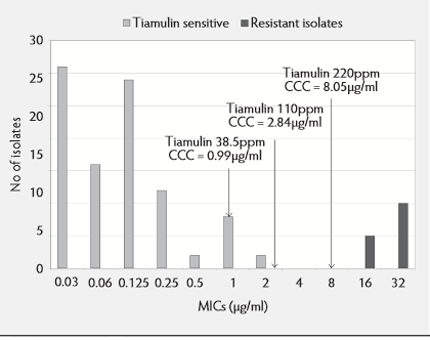
Tiamulin at 38.5ppm in feed can be expected to be inhibitory to most of the 'wild type' isolates up to 0.5 µg/ml. A trial9 demonstrated a complete inhibitory effect at 25 to 40 ppm tiamulin in feed against an isolate of B.hyodysenteriae with an MIC of 0.05µg/ml. At tiamulin 110ppm in feed, a bactericidal effect against 'wild type' isolates might be expected as the MBC:MIC ratio is approximately 2:12 and an inhibitory effect against the first-step mutants.
A similar effect could be expected with water medication at 60ppm and a strong bactericidal, eliminatory effect with tiamulin at 60ppm in the water for three to five days against an isolate with an MIC of 0.05µg/ml9 was shown and at 100ppm and above in feed for seven to 14 days against an isolate with an MIC of 0.5µg/ml10. Concentrations of tiamulin in the colon following 220ppm would expect to achieve a bactericidal effect against both wild type and first-step mutants and could be considered to be achieving a mutant prevention concentration, which should limit resistance development.
Conclusions
An understanding of the PK and PD relationships can help assist the practitioner to decide which drug to use and at what concentration/dose to achieve the optimum results regarding treatment and in the case of swine dysentery, even eradication. It is just possible, with the use of mutant prevention concentrations of tiamulin, that resistance development can be slowed or even halted.
Regarding ileitis, it would appear that some isolates are demonstrating reduced susceptibility even possible resistance to some antibiotics, so again care in selection of the right antibiotic at the right dose to get a good and quick response is increasingly important.
References
1. Anderson, M.D., Stroh, S.L. and Rogers,S. 1994. Tiamulin (Denagard®) activity in certain swine tissues following oral and intramuscular. Proceedings AASP meeting, Chicago, Illinois, USA, pp115-118.
2. Buller, N.B. and Hampson, D.J. 1994. Antimicrobial susceptibility testing of Serpulina hyodysenteriae. Australian Veterinary Journal, 71(7):211-214.
3. Burch, D.G.S. 2005. Pharmacokinetic, pharmacodynamic and clinical correlations relating to the therapy of Lawsonia intracellularis infections, the cause of porcine proliferative enteropathy ('ileitis') in the pig. Pig Journal, 56:25-44.
4. Harding, J., Chirino-Trejo, M., Fernando, C., Jacobson, M., Forster, Z. and Hill, J.E. 2010. Detection of a novel Brachyspira species associated with haemorrhage and necrotizing colitis. Proceedings of the 21st IPVS Congress, vol.2, p740.
5. Karlsson, M., Oxberry, S.L. and Hampson, D.J. 2002. Antimicrobial susceptibility testing of Australian isolates of Brachyspira hyodysenteriae using a new broth dilution method. Veterinary Microbiology, 84:123-133.
6. McOrist, S., Mackie, R.A. and Lawson, G.H.K. 1995. Antimicrobial susceptibility of Ileal Symbiont intracellularis isolated from pigs with Proliferative Enteropathy. Journal of Clinical Microbiology, 33(5):1314-1317.
7. McOrist, S. and Gebhart, C.J. 1995. In vitro testing of antimicrobial agents for proliferative enteropathy (ileitis). J. Swine Health and Production, 3(4):146-149.
8. McOrist, S., Smith, S.H., Shearn, M.F.H., Carr, M.M. and Miller, D.J.S. 1996. Treatment and prevention of porcine proliferative enteropathy with oral tiamulin. Veterinary Record, 139:615-618.
9. Taylor, D.J. 1980. Tiamulin in the treatment and prophylaxis of experimental swine dysentery. Veterinary Record, 106:526-528.
10. Taylor, D.J. 1982. In feed medication with tiamulin in the treatment of experimental swine dysentery. Proceedings of the 7th IPVS Congress, Mexico City, Mexico, p47.
11. Walter, D., Knittel, J., Schwarz, K., Kroll, J. and Roof, M. 2001. Treatment and control of porcine proliferative enteropathy using different tiamulin delivery methods. J. Swine Health and Production, 9(3):109-115.
12. Wattanaphansak, S., Singer, R.S. and Gebhart, C.J. 2009. In vitro antimicrobial activity against 10 North American and European Lawsonia intracellularis isolates. Veterinary Microbiology, 134:305-310.
Further ReadingFind out more information on the diseases mentioned in this article by clicking here. |
April 2013








