



High prevalence of PCV2 recombinants highlights importance of broad vaccine coverage
A recent analysis of porcine circovirus type 2 (PCV2) genetic sequences shows that up to 25% of field strains are recombinants of diverse genotypes, highlighting the importance of broad protection when selecting a PCV2 vaccine.1
“Vaccination is perhaps the most effective tool for managing PCV2, which remains a cause of substantial loss in the swine industry,” said Meggan Bandrick, DVM, PhD, research scientist, Zoetis, and lead investigator of the study.
“However, our analysis confirms that the PCV2 landscape is evolving rapidly and includes a high percentage of recombinants, with most including gene segments from PCV2a, b and d. This makes sense since PCV2a, PCV2b and PCV2d are all circulating. Pork producers would therefore be wise to choose a vaccine that provides as much coverage as possible,” she said.
The analysis was based on thousands of PCV2 genetic sequences that Zoetis scientists obtained from GenBank, the National Institute of Health’s genetic-sequence database. Looking at 3,463 complete and partial PCV2 sequences reported from 2002 to 2016, they found the prevalence of each genotype changed markedly over time.
PCV2a and PCV2b were more commonly reported before 2011, but since 2014, according to GenBank,2 PCV2d has predominated globally and in the US, she noted.
‘Superclusters’
Dennis Foss, DVM, PhD, research scientist, Zoetis, said that to date, at least six PCV2 genotypes have been described with two additional genotypes proposed.
“Most genotypes can be further organized by cluster. In our analysis, we identified two ‘superclusters,’ which were evidenced by gene flow over time: PCV2b/d and PCV2a,” he said (Figure 1).
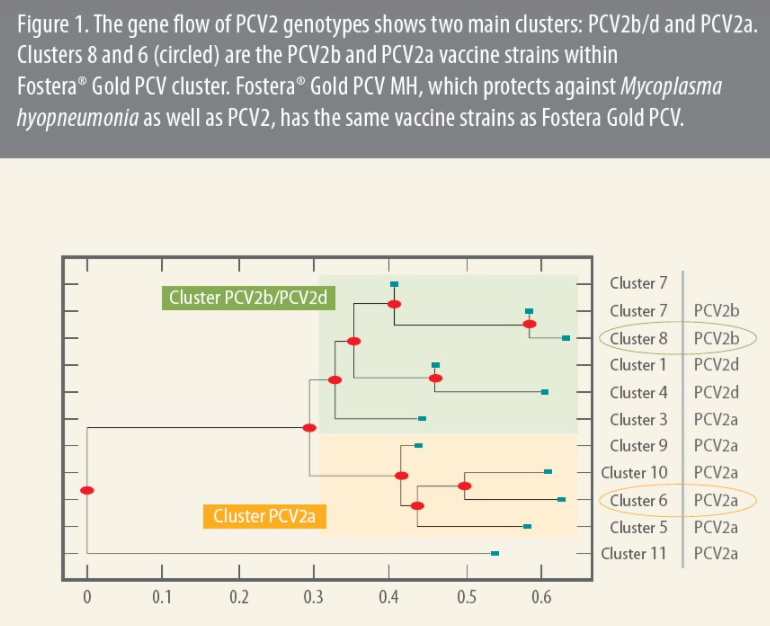
Next, the scientists looked for recombinants using state-of-the-art molecular sequencing techniques, a part of the project led by Gonzalo Rincon, PhD, also a research scientist, Zoetis. Recombination occurs when two viruses co-infect a single cell and generate progeny that share some genetic sequence from both parents.
Of 1,037 full-genome sequences from 41 countries collected between 1980 and 2017, 19.4% were recombinants, with most containing genes from the two superclusters, Rincon said.
“We further analyzed a subset of 161 sequences chosen because they were a manageable number that represented the major clusters of PCV2 across time and geographies,” he said. “Of these, 25% (40) were found to be recombinants, confirming findings by other investigators that recombinants account for 20% to 35% of all PCV2 viruses. And once again, most of the recombinants in our analysis were comprised primarily of the predominant circulating genotypes — 2a, 2b and 2d.”3,4
Broad antigenic coverage
According to Bandrick, the high percentage of recombinant viruses containing genes from both superclusters reinforces the importance of broad coverage when selecting a PCV2 vaccine.
Previously, scientists from Zoetis tested the antigenic coverage of three commercial vaccines to determine which had the best potential for broad protection against recombinant PCV2 field strains.5
In that study, Bandrick and colleagues looked at T-cell epitopes, the part of the viral antigen that is recognized by T cells. T cells play a vital role in immune response by killing infected cells, including those infected with PCV2, Bandrick explained.
Using a computerized process called EpiCC, the scientists characterized T-cell epitopes for the same, globally representative subset of 160 PCV2 sequences that was used in the genetic analysis. They then tested three commercial PCV2 vaccines — Fostera® Gold PCV, which contains PCV2a and PCV2b, and two commercial vaccines containing only PCV2a — for the number and quality of T-cell epitopes they had in common with these sequences.
“With EpiCC, we plotted the coverage of the vaccines against the predominant PCV2 genotypes, which enables us to predict the immune response of different vaccines to the viruses pigs will be exposed to in the field. The analysis showed us that of the three vaccines we tested, Fostera Gold PCV had the most T-cell epitopes in common with the recombinant T-cell epitopes, including recombinant strains from the US,” Bandrick reported.
“This epitope overlap — combined with the fact that it is the only commercial vaccine that covers genotypes from both superclusters — demonstrates the vaccine’s potential to provide the broadest possible coverage against genetically diverse field strains.”
| References | ||||
|---|---|---|---|---|
| 1 Data on file, Study Report Zoetis WO1, EpiCC PCV2 Analysis, Study No. B820Z-US-18-987, Zoetis LLC. | ||||
| 2 Sayers, EW, et al. 2020. GenBank. Nucleic acids research 48, D84-D86. | ||||
| 3 Franzo G, et al. Porcine circovirus 2 (PCV-2) genotype update and proposal of a new genotyping methodology. 2018. PLoS ONE 13 (12): e0208585. | ||||
| 4 Franzo G, et al. Phylodynamic analysis of porcine circovirus type 2 reveals global waves of emerging genotypes and the circulation of recombinant forms. Mol Phylogen Evol 2016;100:269-280. | ||||
| 5 Data on file, Study Report Zoetis WO1, EpiCC PCV2 Analysis, Zoetis LLC. | ||||









