Fostera® Gold PCV MH vaccine is safe for sows and gilts in all stages of gestation
Learn more about how Fostera Gold PCV MH vaccine affects swine gestationPorcine circovirus (PCV2) and Mycoplasma hyopneumoniae (Mhp) vaccination offers many benefits to the breeding herd including a reduction of PCV2 circulation and homogenizing herd immunity. However, it’s important to first demonstrate that porcine circovirus Type 2 (PCV2) and Mycoplasma hyopneumoniae (Mhp) vaccine is safe when used in different stages of gestation.
Dr. Cristina Venegas-Vargas, a member of the Veterinary Medicine Research and Development team at Zoetis, spoke to The Pig Site’s Sarah Mikesell at the Leman Swine Conference in St. Paul, Minnesota about the results of a recent study that tested the safety of Zoetis’ trivalent vaccine containing inactivated PCV2a, PCV2b, and Mhp.
“We conducted a study with Fostera® Gold PCV MH, and the purpose was to evaluate the safety of the vaccine when applied to pregnant swine,” said Dr. Venegas-Vargas.
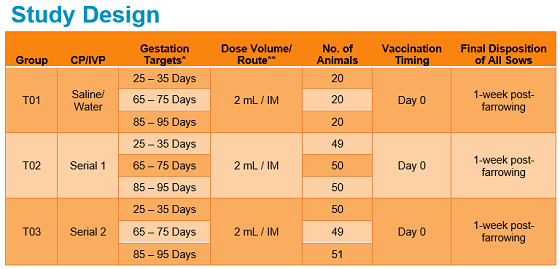
The study enrolled 64 gilts and 296 sows across two commercial farms in the US, ranging in parity from 0 to 7. The gilts and sows received a 2 ml dose of Fostera Gold PCV MH intramuscularly (IM).1
“They were vaccinated at three different stages of gestation, so we looked at the animals from that point all the way through one-week post-farrowing,” she said. “We also observed the piglets that came from those sows during their first seven days.”
The study looked at adverse events in the sows and gilts, injection site reactions, and reproductive and farrowing outcomes as well as litter health during the piglets’ first week of life.
Study results
“There were no observed injection site reactions,” she noted. “The adverse events we observed were classified as either not related to the investigational product (IVP) or unlikely to be related to the IVP.”
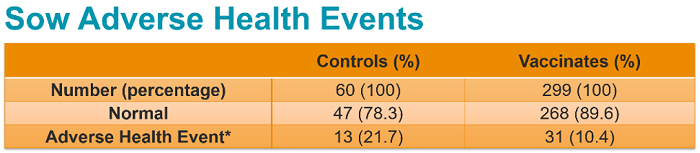
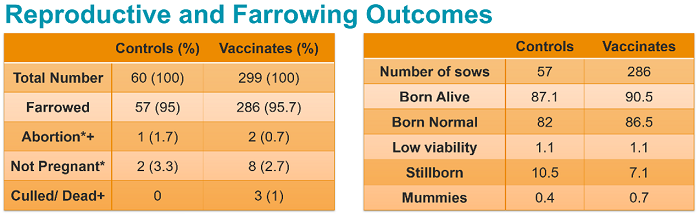
No clinical or biologically relevant differences between the vaccinated groups and the control groups were observed in terms of reproductive outcomes, farrowing outcomes, adverse events or productivity. More than 90% of piglets per litter were normal for each group on each day observed.
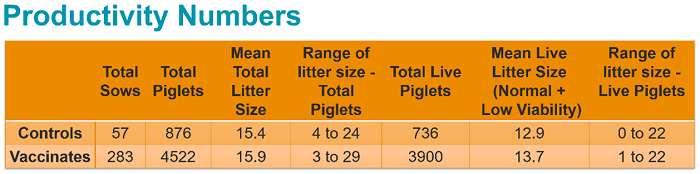
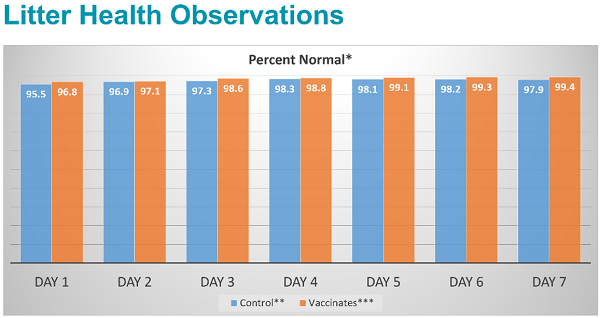
Study conclusion
The data captured during this study confirms the safety of Fostera Gold PCV MH when administered as a single 2 mL dose IM to pregnant swine in all stages of gestation under field conditions.
“Producers can feel comfortable administering the vaccine to protect against PCV2 and Mhp. Now they can also feel confident that the vaccine is safe for pregnant sows and gilts, and it’s also safe for piglets being born from those dams,” said Dr. Venegas-Vargas.