



Enable Right Result First Time by Adding VetMAX Xeno IPC to qPCR Workflows
Recommended in the American Association of Veterinary Laboratory Diagnostics (AAVLD) guidelines, VetMAX Xeno IPC offers veterinary labs rapid, reliable, and easy-to-use solutions for qualifying the effectiveness and accuracy of their qPCR workflows.The Applied Biosystems™ VetMAX™ Xeno™ Internal Positive Control (IPC) Assay is used to monitor the post-lysis sample preparation process and provide a control for the recovery of nucleic acid throughout the sample preparation protocol. This is achieved by spiking in a known copy number of the IPC into the sample lysis reaction and analyzing downstream PCR data by comparing recovery to a negative extraction control.
The Xeno™ assay has been developed to detect the Xeno™ IPC (RNA or DNA) in a background of host and pathogen nucleic acids. The assay consists of primers and probes at limiting concentrations, which can be multiplexed with a primary target assay. Since the IPC is only meant to serve as a control, it is important to make sure that the Xeno assay does not interfere with the detection of the primary target nucleic acid. The Xeno assay has been designed to be specific for only the Xeno IPC (RNA or DNA).
Guidelines for using the Xeno IPC (sample prep)
The Xeno IPC has been tested at a concentration of 20,000 copies/reaction in volumes between 130 μL and 700 μL of lysis solution. This concentration ensures that the assay is sensitive enough to detect the Xeno IPC at a consistent Ct range in normal (i.e., noninhibited) samples. Using Applied Biosystems™ reagents and workflows, the expected range of Xeno IPC detection is 27–34 Ct.
In order to optimize Xeno IPC for your particular sample preparation, the following must be considered.
- The Xeno IPC is provided at 10,000 copies/μL and spiking 2 μL (20,000 copies) into each reaction is recommended. This is based on a lysis solution volume between 130 μL and 700 μL. If your lysis solution volume is greater than 1 mL/reaction, please consider adding more Xeno IPC to compensate for the dilution factor. As a general rule, 20,000 copies/mL of lysis solution should suffice.
- The Xeno IPC is made up of unprotected nucleic acids. This means that it does not have a protein envelope to protect it from nucleases that are present in sample matrices and is thus susceptible to degradation. In order to maintain the integrity of the Xeno IPC, it must be spiked into a solution containing a protector of nucleic acids, such as a guanidinium–based lysis solution.
- The Xeno IPC is meant to be susceptible to inhibition. If the sample matrix contains high concentrations of inhibitors, the Xeno IPC may not amplify. An effective nucleic acid isolation and purification method will reduce the impact of inhibition.
Guidelines for using the Xeno assay (real-time PCR)
In order to optimize the Xeno assay for your particular real-time PCR needs, consider the following:
- The Xeno assay is designed to be multiplexed with your target assay. It should not interfere with the sensitivity of your target assay.
- The Xeno assay can be purchased with either a VIC™ or LIZ™ reporter dye. This provides some flexibility based on the primary target dye in the multiplex.
- The Xeno assay is provided as a 25X mix so each 25 μL reaction includes 1 μL of Xeno assay.
Optimizing your Xeno multiplex assay
The following are recommendations on how to optimize multiplexing your target assay with the Xeno assay. Please note that the same procedure can be followed when optimizing any multiplex assay.
- Prepare a 5–8 point standard curve with your target RNA or DNA, making sure to have enough dilution points to cover the concentrations around the limit of detection (LOD) for your target assay. For example, if the LOD is 50 copies/reaction, you want to have dilutions at 100 copies/reaction, 50 copies/reaction, and 25 copies/reaction. Each dilution point is tested in triplicate.
- In order to determine whether the Xeno assay adversely affects the target assay sensitivity when multiplexed, test this standard curve with and without the Xeno assay multiplexed with your target assay. The following points have to be taken into consideration when performing this experiment.
- It is recommended to add Xeno IPC to each standard curve reaction at a set concentration so that the effect of a secondary target (i.e., Xeno IPC) can be determined. In order to determine the set concentration to be added to each standard curve reaction, first calculate the maximum copy number of Xeno IPC, which can be expected in the elution fraction from the sample prep. For example, if 2 μL of Xeno IPC are spiked into each sample prep reaction, and eluted in 80 μL, a maximum concentration of 250 copies of Xeno IPC/μL can be expected (2 μL x 10,000 copies/μL = 20,000 copies; 20,000 copies/80 μL = 250 copies/μL).
- Next, to ensure consistency of the Xeno IPC spike and ease of setup, it is recommended to add the Xeno IPC directly to the PCR mastermix. Using the above example, a typical PCR reaction with 5 uL of sample input would yield a final concentration of 1,250 copies/reaction of Xeno IPC (250 copies μL x 5 μL = 1,250 copies/reaction).
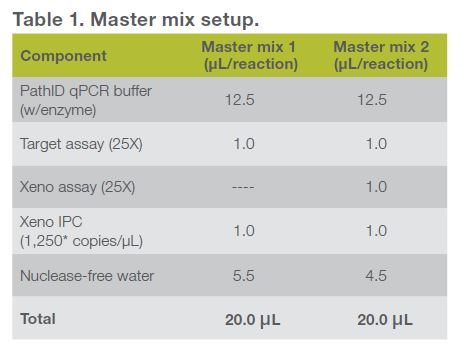
- From the Xeno IPC stock supplied at 10,000 copies/μL, prepare a 1:8* dilution in nucleic acid dilution buffer to produce a working stock concentration of 1,250* copies/μL. From this working stock solution of Xeno IPC, add 1 μL to each reaction of your master mix for a final concentration of 1,250* copies/reaction of Xeno IPC. Follow the master mix recipe below (Table 1) as a guideline for reaction setup.
* Number may vary based on calculations from 2a and 2b.
- Make two separate master mixes according to Table 1. One master mix will contain the target-Xeno assay multiplex and one will contain only the target assay.
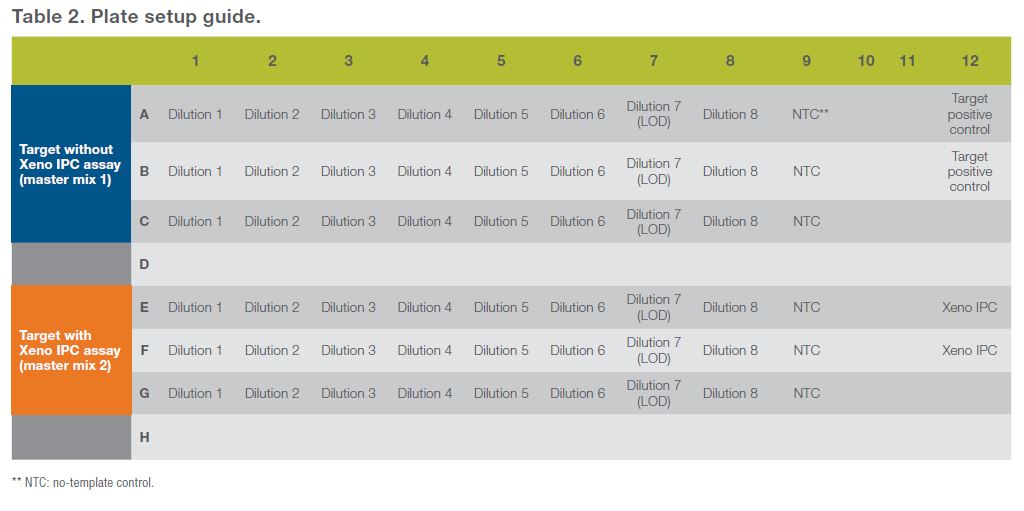
- Add 5 μL of each of your target standard curve dilutions to 20 μL of master mix in each well, according to the plate setup (Table 2). Add 5 μL of Xeno IPC diluted to 1,000 copies/μL as your Xeno IPC positive control, 5 μL of your target positive control, and 5 μL of nuclease-free water to your NTC (no-template control) as indicated in Table 2.
- Run qPCR, using the thermal profile recommended for your assay.
Analysis
- Determine the threshold setting for each target dye based on your assay protocol.
- For the Xeno assay, we recommend setting the threshold to 10% of the maximum delta Rn (ΔRn) of the Xeno positive PCR control diluted to 1,000 copies/μL. For example, if the maximum ΔRn value of the Xeno positive PCR control is 2.45, then set the threshold at 0.245.
- Compare the sensitivity (number of dilutions detected) with and without the multiplex of your target assay and the Xeno assay. The sensitivity of the target assay should remain the same in the presence of the Xeno assay; the LOD should be equivalent between the two master mixes.
- Check the negative controls (NTC) to make sure there is no nonspecific amplification produced when the target and Xeno assays are multiplexed.
- If the results are satisfactory, you can test the target and/or Xeno assay multiplex with field samples to confirm usability. If not, please refer to the troubleshooting section in the Xeno IPC and Assay instructions for use.
Troubleshooting
Please refer to the Xeno IPC and Assay instructions for product troubleshooting.
Find out more at thermofisher.com/xeno-ipc
For more information about swine diagnostics, click here or connect to the Thermo Fisher Scientific Swine Resource Center.









