



Bordetella bronchiseptica and respiratory disease in swine: an overview
This overview offers producers and veterinarians guidance on the identification, treatment and prevention of Bordetella bronchiseptica and respiratory disease in swine.Abstract
Bordetella bronchiseptica is an ubiquitous pathogen of swine causing respiratory infections including rhinitis, tracheitis, bronchitis, and pneumonia. It spreads quickly between animals, especially during commingling of naïve animals with subclinical carriers. Bordetella bronchiseptica causes disease through the production of virulence factors, such as adhesins and toxins, which is coordinated by the BvgAS system. In addition to causing primary disease, Bordetella bronchiseptica can exacerbate viral respiratory infections and predispose animals to other bacterial respiratory pathogens, which increases the clinical significance of Bordetella bronchiseptica colonization. Disease is currently managed with antibiotic treatments and vaccination strategies; however, colonization is difficult to clear and animals can remain colonised and shed Bordetella bronchiseptica long term, acting as a source of infection for naïve animals within a herd.
Diseases
Bordetella bronchiseptica causes respiratory disease in swine that can impact both the upper and lower respiratory tracts. Bordetella bronchiseptica is associated with a high morbidity and low mortality, and disease has a rapid onset, with clinical signs developing 2-3 days post-exposure.
Bordetella bronchiseptica is the causal agent of Non Progressive Atrophic Rhinitis, and it causes tracheitis, bronchitis and pneumonia.
Bordetella bronchiseptica is a primary cause of rhinitis in pigs, which can present as mild rhinitis, nonprogressive atrophic rhinitis, when Bordetella bronchiseptica is the sole etiologic agent, or progressive atrophic rhinitis, when Bordetella bronchiseptica infects with toxigenic Pasteurella multocida. The interaction of Bordetella bronchiseptica with the nasal mucosa leads to inflammation (rhinitis). Over time, disease progresses to atrophic rhinitis, in which the boney trabeculae of the turbinates is replaced with fibrous connective tissue through the action of the dermonecrotic toxin (DNT). With Bordetella bronchiseptica as the sole etiologic agent, the disease is nonprogressive, affecting predominantly the ventral scrolls and turbinates, which can take weeks to resolve; however, when Bordetella bronchiseptica interacts with toxigenic strains of Pasteurella multocida, disease is more severe. The Pasteurella multocida toxin (PMT) and DNT act synergistically to cause more severe atrophy of the turbinates, which can result in septal deviation. Rhinitis presents with clinical signs of sneezing, nasal discharge, and ocular discharge. As atrophic rhinitis progresses, atrophy of the turbinates can be visualised at necropsy and during coinfection with Pasteurella multocida, can lead to brachygnathia, lateral deviation of the snout (Figure 1), and/or epistaxis.

Fattening pig with lateral deviation of the snout. © HIPRA.
Bordetella bronchiseptica is also capable of causing inflammation of the airways resulting in tracheitis and bronchitis. This reduces the clearance by the mucociliary escalator causing accumulation of mucus in the airways, which may provide a niche for secondary bacteria and predispose the animal to pulmonary infection.
Pulmonary infection with Bordetella bronchiseptica results in suppurative bronchopneumonia (Figure 2). It can act as the primary etiologic agent in young pigs and in older pigs it generally contributes to the severity of the Porcine Respiratory Disease Complex (PRDC) through interaction with other bacteria and viruses in mixed infections. The necrosis and tissue damage associated with Bordetella bronchiseptica pneumonia is induced by the action of DNT, without which pneumonia does not develop. The most prevalent clinical sign of Bordetella bronchiseptica pneumonia is coughing and it can be difficult to discern the cause without further diagnostics.
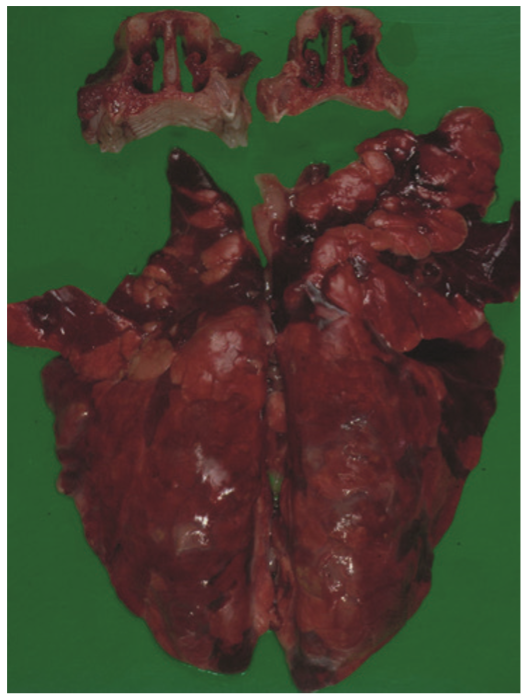
Turbinate and lung lesions in a nursery pig affected by Bordetella bronchiseptica.© Histology and Anatomical Pathology, Faculty of Veterinary Medicine, University of Murcia.
Bordetella bronchiseptica is a predisposing factor for other diseases.
Bordetella bronchiseptica is also capable of increasing the severity of respiratory disease caused by viral pathogens and predisposing pigs to secondary infection with other bacterial pathogens. Disease associated with Porcine Reproductive and Respiratory Syndrome Virus (PRRSV), Influenza A Virus (IAV), and Porcine Respiratory Corona Virus (PRCV) is exacerbated by coinfection with Bordetella bronchiseptica. Bordetella bronchiseptica was found to increase lesion severity and prolong disease resolution when contributing to a viral respiratory infection, which may be associated with elevated and sustained cytokine response during coinfection of viral agents and Bordetella bronchiseptica. Bordetella bronchiseptica has also been shown to enhance colonization and disease with other bacterial agents. The association between Bordetella bronchiseptica and Pasteurella multocida has been well studied as it relates to progressive atrophic rhinitis. Additionally, Pasteurella multocida is less capable of causing pneumonic lesions as the sole agent or during PRRSV coinfection; however, when Pasteurella multocida is associated with PRRSV and Bordetella bronchiseptica, more severe pneumonia develops. Bordetella bronchiseptica also predisposes animals to disease with Streptococcus suis, promotes colonization with Haemophilus parasuis, and may contribute to the persistence of Actinobacillus pleuropneumoniae in the respiratory tract.
Although Bordetella bronchiseptica is ubiquitous within the swine industry, this organism is not a commensal and the differences in severity of Bordetella bronchiseptica disease are associated with the age and immune status of the animal, environmental conditions, coinfections, and variations in Bordetella bronchiseptica isolates. Younger animals tend to develop more severe nasal and pulmonary lesions. Bordetella bronchiseptica disease is also impacted by immune status and passive immunity, with piglets from vaccinated sows having delayed onset and/or reduced severity of lesions. Environmental conditions, such as poor air quality, can exacerbate disease with Bordetella bronchiseptica as well as coinfections with other pathogens. Differences in isolate virulence also contribute to differences in disease presentation and a broad range of virulence has been detected (lethal dose 50 variation of 100,000 fold in inbred mice). However, even with less virulent isolates, Bordetella bronchiseptica predisposes animals to secondary infections and, when present, Bordetella bronchiseptica should not be considered a commensal.
Pathogenesis
Bordetella bronchiseptica infection begins with attachment of the bacterium to the respiratory epithelium, with preferential adherence to ciliated cells in the mucosa. Through this interaction and toxin production, Bordetella bronchiseptica causes ciliostasis and the extrusion of ciliated epithelial cells, which contributes to mucus accumulation within the respiratory tract. Bordetella bronchiseptica causes inflammation of the epithelium, leading to epithelial metaplasia and necrosis and the infiltration of immune cells, which Bordetella bronchiseptica combats through being cytotoxic to phagocytes. This allows the bacterium to persist during tissue inflammation and evade the host immune response. Additionally, while they are not the preferred cell type, the interaction of Bordetella bronchiseptica with nonciliated epithelial cells is thought to play a role in establishing microcolonies or biofilms, especially within the nasal cavity. Biofilms are communities of microorganisms within a complex matrix that function in adherence, protect the organisms from the environment, and contribute to persistent infections in bacteria such as Bordetella bronchiseptica.
The pathogenesis of Bordetella bronchiseptica infection is associated with the coordinated implementation of various virulence factors that are regulated by the BvgAS (Bordetella virulence gene) system. This system functions as an on/off switch regulating the virulence genes of Bordetella bronchiseptica and enabling phenotypic modulation that allows the bacterium to succeed as it cycles between the environment and the host. The Bvg+ state is induced by an elevation in temperature during the transition of Bordetella bronchiseptica from the environment to infecting a host. The virulence factors induced by the BvgAS system are generated in two phases: “early” and “late”. Early genes induced by BvgAS function predominantly in adherence and aid the bacterium in establishing host infection. Late genes induced by BvgAS include toxins and virulence factors which cause tissue damage and are involved in host evasion. One prominent toxin is DNT, which is essential for the bony changes associated with nonprogressive atrophic rhinitis and the necrosis associated with pneumonia. At environmental temperatures in the Bvg- state, Bordetella bronchiseptica has elevated expression of genes involved in motility and urease production, while virulence factors are repressed.
Transmission
Bordetella bronchiseptica is highly infectious and spreads rapidly throughout a herd to infect most animals. The bacterium spreads through direct contact between infected sows and their piglets as well as between pigs after comingling. Bordetella bronchiseptica can also spread via aerosols between animals without direct contact, which is promoted by the sneezing and coughing associated with the progression of disease. After acute disease resolves, Bordetella bronchiseptica colonization persists for several months and infected animals can serve as a reservoir for newly introduced, naïve animals. Bordetella bronchiseptica is also able to persist in the environment with a half-life of 1-2 hours at ambient temperatures and 75 percent relative humidity and is known to survive weeks in soil or water, which indicates fomites may also play a role in transmission.
Diagnostics
Bordetella bronchiseptica grows readily on blood agar plates; however, because Bordetella bronchiseptica is a slower-growing organism, it can be difficult to identify without the use of a more restrictive media to prevent overgrowth of contaminating bacteria. Bordetella bronchiseptica can be detected through isolation and biochemical identification from nasal swabs, or in cases of pneumonia from lung tissue samples or washes from pigs with pneumonia. Additionally, a polymerase chain reaction (PCR) target has been developed that has shown 100 percent specificity and sensitivity for Bordetella bronchiseptica. More recently the use of oral fluids has compared favorably to the use of nasal swabs for detecting Bordetella bronchiseptica via PCR at the herd level, improving the ease of sample collection. Collection cards that lyse the bacteria and capture and stabilise the DNA for transport and storage at room temperature have also been used successfully to detect Bordetella bronchiseptica in oral fluids. Atrophic rhinitis prevention programs can also be assessed using a snout scoring system, in which turbinate atrophy is quantified using a transverse sectioned snout (Figure 3).
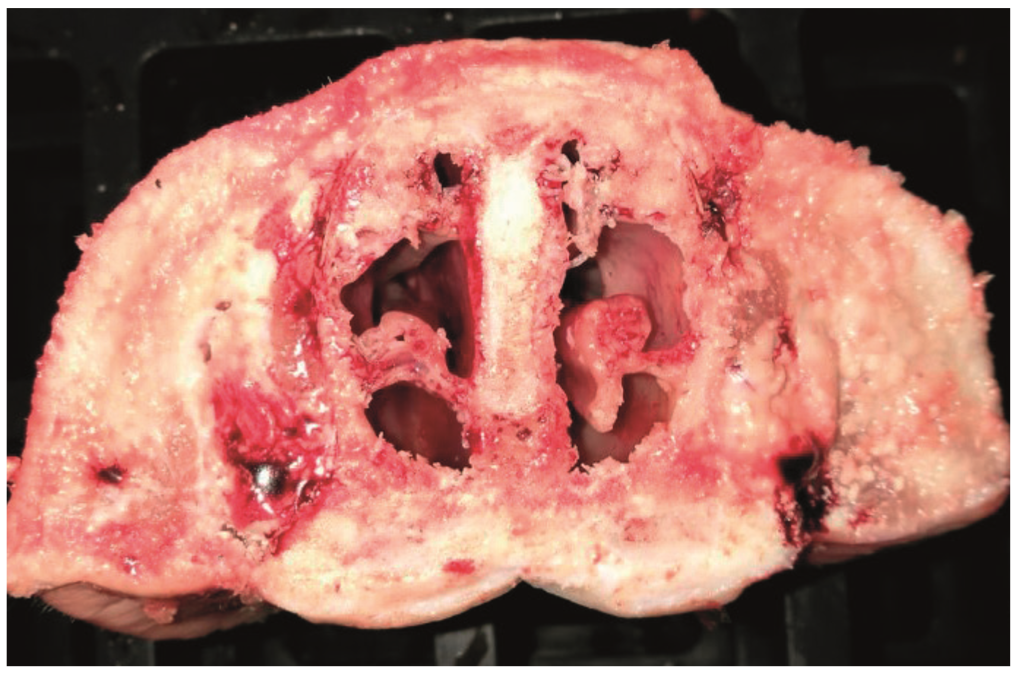
Routine evaluation of the nasal turbinates in the slaughterhouse and in nursery animals is now the best way of confirming or ruling out Progressive or Non-Progressive AR, respectively. © HIPRA
Treatment and prevention
Control of Bordetella bronchiseptica has been accomplished primarily with antibiotic treatments and vaccination to develop protective immunity. Bordetella bronchiseptica tends to be susceptible to chlortetracycline, oxytetracycline, and enrofloxacin, all antibiotics approved for the treatment of bacterial agents associated with PRDC, but not specifically Bordetella bronchiseptica. While antibiotics have been useful in reducing pneumonia and clinical signs associated with Bordetella bronchiseptica infection, they are not effective in clearing the upper respiratory tract of infection and animals continue to shed Bordetella bronchiseptica after treatment. In addition, the trend to reduce antibiotic usage in animal production in general, and more specifically as a disease prevention measure, may result in a reemergence of overt atrophic rhinitis lesions in herds.
Vaccination as prevention and control of AR
Vaccination against Bordetella bronchiseptica in swine typically utilises whole cell bacterins often including inactivated Pasteurella mutocida toxin to provide better control of progressive atrophic rhinitis. Although vaccination with bacterins does not provide sterilising immunity, it can prevent clinical signs, lower bacterial numbers, and limit the severity of disease with Bordetella bronchiseptica.
Bacterins for sow vaccination protocols involve vaccinating animals pre-farrow and rely on passively acquired maternal immunity to limit the severity of disease in piglets. Reduction of the prevalence of infection, bacterial load, and severity of nasal lesions, as well as increased weight gains have been demonstrated in piglets from Bordetella bronchiseptica bacterin vaccinated sows. It is reasonable to suggest that vaccination against Bordetella bronchiseptica may also reduce infection or severity of disease with secondary pathogens, nevertheless additional research into the effectiveness of vaccination in preventing enhanced disease with coinfection models using Bordetella bronchiseptica and other respiratory pathogens is needed.
Conclusion
Bordetella bronchiseptica is a widespread, highly infectious cause of respiratory disease in swine. Disease varies from mild, subclinical infections to severe pneumonia or atrophic rhinitis. The presence of mild and subclinically infected animals causes Bordetella bronchiseptica to be an underreported contributor to respiratory disease in pigs. Bordetella bronchiseptica also exacerbates viral respiratory infection and enhances colonisation and/ or disease with other bacterial pathogens, which makes the full impact of Bordetella bronchiseptica on swine respiratory health difficult to discern. Vaccination can improve clinical health and improve performance, but control of Bordetella bronchiseptica can be challenging due to the difficulty in fully clearing the bacterium using vaccines and antibiotics.
The article was prepared by a US Department of Agriculture employee as part of his/her official duties. Articles and other publications prepared as part of a Federal employee’s official duties are property of the US Government. Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture. This article has been done in collaboration with LABORATORIOS HIPRA, S.A.
Access the article online for a full list of references.







